- Department of Neurosurgery, Houston Methodist Hospital, Houston, Texas, United States
- Department of Pathology, Houston Methodist Hospital, Houston, Texas, United States.
Correspondence Address:
Samuel K. Asante, Department of Neurosurgery, Houston Methodist Hospital, Houston, Texas, United States.
DOI:10.25259/SNI_231_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Samuel K. Asante1, Jonathan J. Lee1, Amanda V. Jenson1, Lokeshwar S. Bhenderu1, John D. Patterson1, Andreana L. Rivera2, Gavin W. Britz1. Primary thoracic intramedullary spinal cord tumor with likely metastases of glial origin to the lumbosacral vertebrae: Illustrative case. 15-Sep-2023;14:333
How to cite this URL: Samuel K. Asante1, Jonathan J. Lee1, Amanda V. Jenson1, Lokeshwar S. Bhenderu1, John D. Patterson1, Andreana L. Rivera2, Gavin W. Britz1. Primary thoracic intramedullary spinal cord tumor with likely metastases of glial origin to the lumbosacral vertebrae: Illustrative case. 15-Sep-2023;14:333. Available from: https://surgicalneurologyint.com/surgicalint-articles/12554/
Abstract
Background: Metastasis of systemic neoplasms to the spine is common; however, the metastasis of primary spinal cord tumors to other regions in the body is an infrequent occurrence. A few case reports have described the metastasis of primary spinal cord tumors, and in most cases, patients were younger than 30 years of age.
Case Description: We present an illustrative case of a 47-year-old female with metastatic lesions to the lumbosacral vertebrae years after the initial diagnosis of an intradural, intramedullary spinal cord tumor (IMSCT). Although the surgical biopsy of the IMSCT was nondiagnostic, the patient was not found to have a separate primary neoplastic source, and the specimens of the metastatic lesions from the lumbar vertebral body were of glial origin.
Conclusion: Metastasis from primary IMSCTs is extremely rare. Distant vertebral body and intracranial metastasis are even rarer yet possible. The clinical course is highly aggressive and responds poorly to current standard treatment.
Keywords: Intramedullary, Metastasis, Spinal cord tumor, Vertebral body
INTRODUCTION
Primary spinal cord tumors account for 2–4% of all primary central nervous system tumors, and an estimated 850–1700 new adult cases occur every year in the United States.[
A study of 70 patients with IMSCTs found the median age of presentation to be 41 years.[
Although metastasis of systemic neoplasms to the spine is a common occurrence,[
We present an illustrative case of a 47-year-old female with metastases to the lumbosacral vertebrae years after the initial diagnosis of a T4/5 IMSCT. We also provide a review of the literature on similar cases.
CASE DESCRIPTION
A 47-year-old female with a medical history of hypothyroidism presented in 2016 with months of pain radiating from the posterior mid-thoracic region to the right scapular area and the anterior portion of the right side of the chest. She also experienced right lower extremity paresthesias without complaints of weakness in her extremities. The patient described her pain as a burning-like quality. Her physical examination was unrevealing with full motor strength in her extremities. The initial workup, including a chest X-ray and mammogram, was negative.
Initial imaging
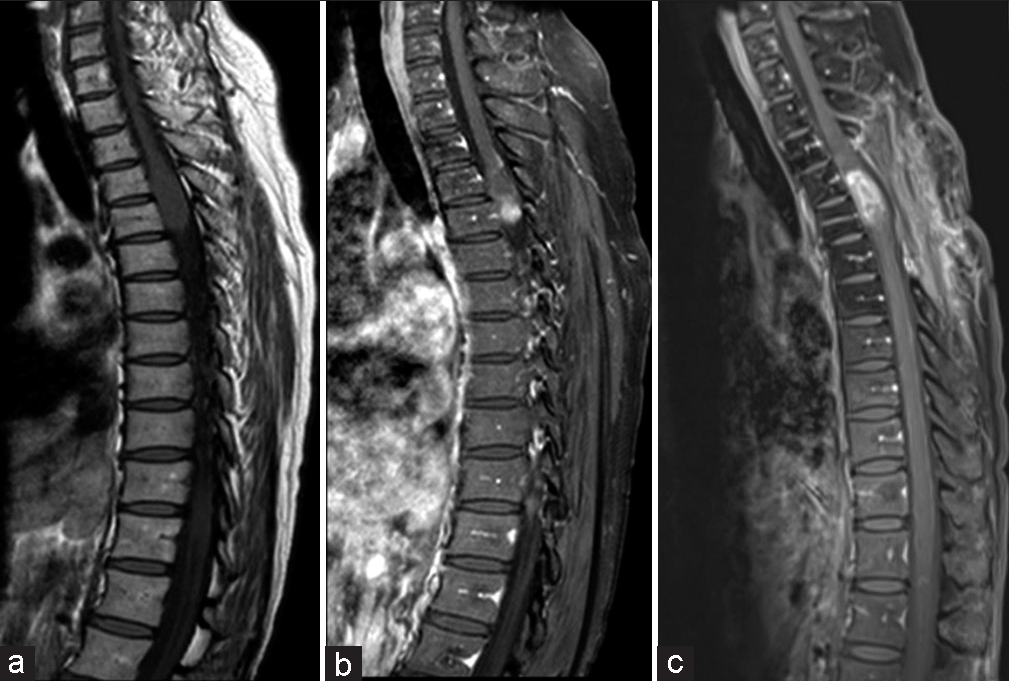
A magnetic resonance imaging (MRI) of the thoracic spine without contrast revealed a spinal cord tumor extending from the superior endplate of T4 to the inferior endplate of T5. There was a considerable enlargement of the spinal cord; the spinal cord with the tumor measured approximately 11 mm in the anterior-posterior dimension and 15 mm in the coronal plane. The imaging findings were concerning for a primary spinal cord neoplasm, such as an ependymoma or astrocytoma [
Initial surgical procedure
The patient was taken to the operating room for tissue diagnosis 1 month after the presentation in 2016. The neurosurgical team performed a T3–6 bilateral laminectomy. Neural monitoring, including motor-evoked potentials (MEPs) and somatosensory-evoked potentials (SSEPs), was used during the case. After the thoracic laminectomies, the tumor could be seen through the dura. The IMSCT caused the spinal cord to look severely swollen. After the dural opening, the tumor was noted to be localized to the left side of the spinal cord, more lateral than had been anticipated based on previous imaging. A paramedian myelotomy was performed in a surgical plane between the tumor and the spinal cord, and several tumor biopsy specimens were taken.
The neuropathology team concluded that the specimen could only be termed “abnormal tissue,” with no other absolute findings for a specific pathology. After the biopsy specimens had been taken, there was a temporary, intermittent decrease in amplitude in the left tibial MEP and a temporary decrease in SSEP signals on the patient’s left side. Due to this decrease in neural monitoring signals, the surgical team decided not to take additional specimens and concluded the operation. The final pathology of the surgical specimens was non-diagnostic. There was evidence of Rosenthal fibers; however, BRAF mutation was absent. After a careful review of the case at the Texas Medical Center multi-institutional Neuropathology consensus conference by multiple-board certified neuropathologists, it was decided not to send the tumor biopsy to another institution.
Postoperative course and treatment
Despite the nondiagnostic final pathology from the thoracic spinal cord lesion, the patient received radiation therapy (a total dose of 54 Gray in 27 fractions) and chemotherapy consisting of four doses of bevacizumab and six cycles of temozolomide at 150 mg/m2 for 5 days every month. Her motor examination continued to remain stable after surgery. In postoperative year 1, a gadolinium-enhanced MRI of the thoracic spine showed possible pseudo-progression [
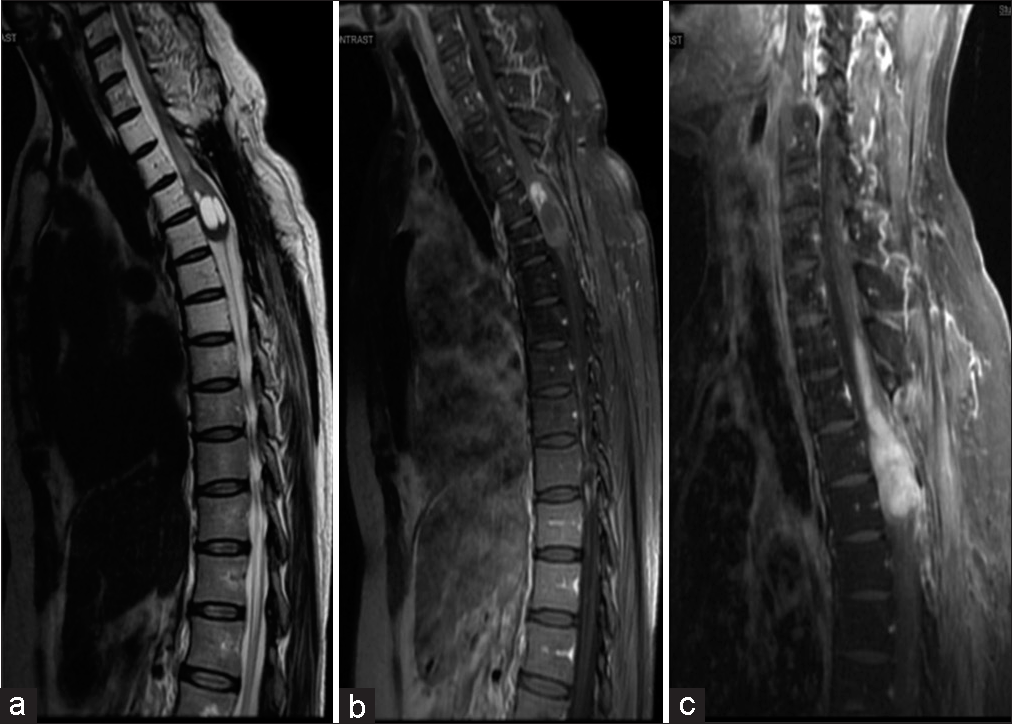
At 20 months post-surgery, the patient complained of new lower extremity paresthesias in a contrasting distribution from her initial presentation. Another gadolinium-enhanced MRI of the thoracic spine showed a large, enhancing nodule superior to the cystic portion of the tumor [
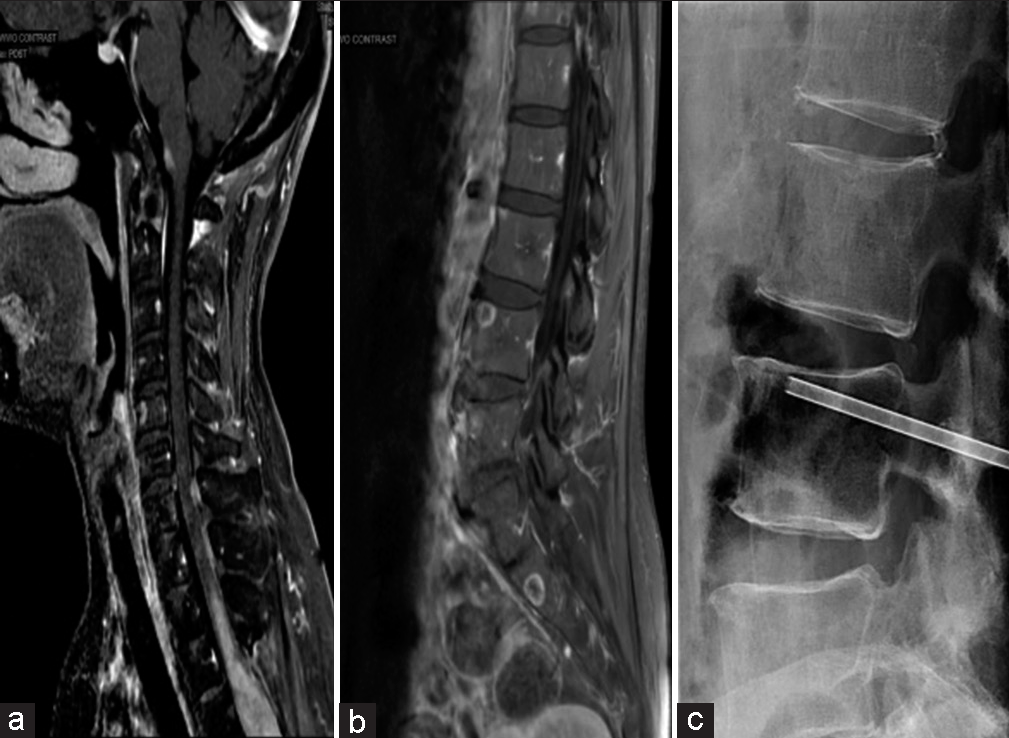
A 2-year postoperative MRI showed a reduction in the size of the cystic component of the tumor; however, a 3-year postoperative MRI showed progression of the thoracic tumor superiorly with extension to the C7-T1 level [
Biopsy of metastases, pathology analysis, and clinical course
Subsequently, the patient underwent a fluoroscopic-guided biopsy of the L4 vertebral body [
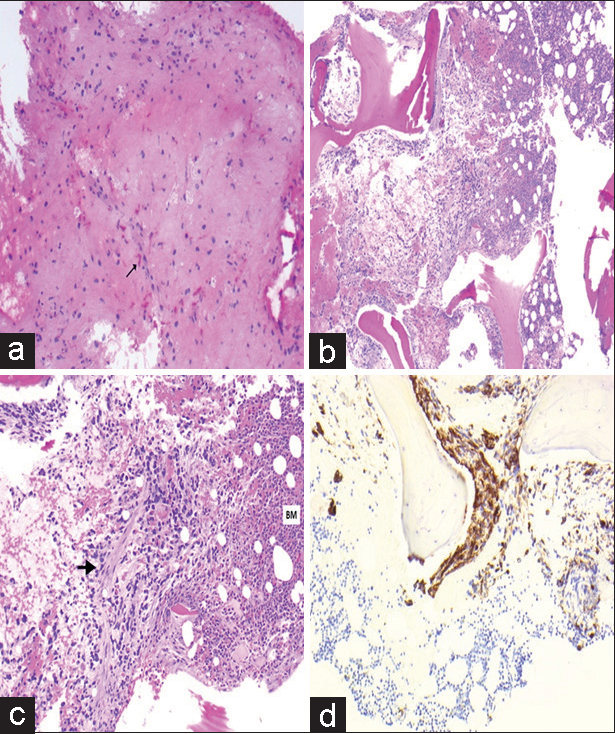
Figure 4:
Pathology images after open surgical biopsy and L4 vertebral biopsy. Pathology images (a) initial open surgical biopsy-hematoxylin and eosin (H&E) stain showing the slightly hypercellular glial tissue with scattered Rosenthal fibers labeled with an arrow. L4 vertebral biopsy: (b) H&E stain ×4– L4 vertebral body biopsy showing glial cells embedded in bone marrow, (c) H&E stain ×10–L4 vertebral body biopsy, and (d) glial fibrillary acidic protein (GFAP) stain-L4 vertebral body biopsy with the brown staining confirming glial tissue. The bone marrow is designated by “BM” in the image with the suspected glial tissue marked with the arrowhead.
The patient underwent palliative radiation of 37.5–45 Gy in 15 fractions to the cervical and lumbar spine. The patient continued to have difficulty ambulating. Her neurologic examination worsened to a motor strength of 2/5 in her lower extremities and to complete loss of her bowel and bladder sensation. After a lengthy discussion, the patient and her family decided to proceed with hospice care.
DISCUSSION
The pathology from the initial surgery of the thoracic IMSCT tumor, although nondiagnostic, had evidence of Rosenthal fibers, which suggested that the tumor was an ependymoma or a pilocytic astrocytoma, among other possibilities. The EMA staining and BRAF mutations, to definitively classify the tumor being either an ependymoma or a pilocytic astrocytoma, were negative. Despite this, the oncology team decided to initiate treatment with radiation and chemotherapy.
In general, the treatment options for IMSCTs include surgical resection, radiation therapy, and chemotherapy. These treatments are often combined, and the exact therapy depends on the tumor’s histology, location, and staging.[
There are currently no long-term and prospective studies that investigate the treatment of IMSCTs with unknown pathology treating primary IMSCTs with unknown pathology. Thus, this patient’s treatment plan was tailored toward treating an aggressive ependymoma or astrocytoma. From the biopsy of the L4 vertebral body, the intermingling of glial cells within the bone marrow suggested the glial tissue had been growing for an extended period [
Metastases from primary IMSCTs are extremely rare, and only a few cases have been reported in the literature. In one publication by Handis et al., a thoracic intramedullary H3K27M mutant glioma was reported to have seeded at the level of the cauda equina, with intracranial dissemination and extensive vertebral body metastasis.[
In the aforementioned cases, metastases from IMSCTs were found at the initial presentation and diagnosis of the IMSCT. In our case, evidence of metastasis to the lumbosacral spine was observed 3 years after the patient’s initial presentation and after many rounds of chemoradiation.
CONCLUSION
Metastases from primary IMSCTs are rare. Distant vertebral body and intracranial metastasis are even rarer yet possible. Their clinical course is very aggressive and responds poorly to current standard treatment.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abel TJ, Chowdhary A, Thapa M, Rutledge JC, Geyer JR, Ojemann J. Spinal cord pilocytic astrocytoma with leptomeningeal dissemination to the brain. Case report and review of the literature. J Neurosurg. 2006. 105: 508-14
2. Aghayev K, Vrionis F, Chamberlain MC. Adult intradural primary spinal cord tumors. J Natl Compr Canc Netw. 2011. 9: 434-47
3. Balmaceda C. Chemotherapy for intramedullary spinal cord tumors. J Neurooncol. 2000. 47: 293-307
4. Bell WO, Packer RJ, Seigel KR, Rorke LB, Sutton LN, Bruce DA. Leptomeningeal spread of intramedullary spinal cord tumors. Report of three cases. J Neurosurg. 1988. 69: 295-300
5. Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: A review. Curr Neurol Neurosci Rep. 2011. 11: 320-8
6. Duffner PK, Cohen ME. Extraneural metastases in childhood brain tumors. Ann Neurol. 1981. 10: 261-5
7. Goodwin CR, Liang L, Abu-Bonsrah N, Hdeib A, Elder BD, Kosztowski T. Extraneural glioblastoma multiforme vertebral metastasis. World Neurosurg. 2016. 89: 578-82.e3
8. Handis C, Tanrıkulu B, Danyeli AE, Özek MM. Spinal intramedullary H3K27M mutant glioma with vertebral metastasis: A case report. Childs Nerv Syst. 2021. 37: 3933-7
9. Harrop JS, Ganju A, Groff M, Bilsky M. Primary intramedullary tumors of the spinal cord. Spine (Phila Pa 1976). 2009. 34: S69-77
10. Jayachandran A, Jonathan GE, Patel B, Prabhu K. Primary spinal cord glioblastoma metastasizing to the cerebellum: A missed entity. Neurol India. 2018. 66: 854-7
11. Joo MS, Rho YJ, Song SW, Koh YC, Roh HG, Lim SD. Metastatic intracranial hemangiopericytoma to the spinal column: A case report. Brain Tumor Res Treat. 2016. 4: 128-32
12. Morais N, Mascarenhas L, Soares-Fernandes JP, Silva A, Magalhães Z, Costa JA. Primary spinal glioblastoma: A case report and review of the literature. Oncol Lett. 2013. 5: 992-6
13. Payer S, Mende KC, Westphal M, Eicker SO. Intramedullary spinal cord metastases: An increasingly common diagnosis. Neurosurg Focus. 2015. 39: E15
14. Samartzis D, Gillis CC, Shih P, O’Toole JE, Fessler RG. Intramedullary spinal cord tumors: Part I-epidemiology, pathophysiology, and diagnosis. Global Spine J. 2015. 5: 425-35
15. Strong MJ, Koduri S, Allison JA, Pesavento CM, Ogunsola S, Ogunsola O. Bone metastasis from glioblastoma: A systematic review. J Neurooncol. 2022. 158: 379-92
16. Tadele A, Woodraw S, Johnson MD. Case report of spinal cord astrocytoma presenting with extensive spinal vertebral body destruction. World Neurosurg X. 2019. 2: 100016
17. Tobin MK, Geraghty JR, Engelhard HH, Linninger AA, Mehta AI. Intramedullary spinal cord tumors: A review of current and future treatment strategies. Neurosurg Focus. 2015. 39: E14
18. Tominaga T, Koshu K, Narita N, Yoshimoto T. Metastatic meningioma to the second cervical vertebral body: A case report. Neurosurgery. 1994. 34: 538-9