Prognosis of spinal infections managed by minimal debridement: A case series in two tertiary centers
- Department of Neurosurgery, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt.
- Department of Neurosurgery, Kasr Alainy Faculty of Medicine, Cairo University, Cairo, Egypt.
Correspondence Address:
Hashem Mohamed Aboul-Ela
Department of Neurosurgery, Kasr Alainy Faculty of Medicine, Cairo University, Cairo, Egypt.
DOI:10.25259/SNI_29_2021
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ahmed Ali Mohamed1, Hussein Mohammed Soffar2, Hazem Hassan El Zayat1, Hashem Mohamed Aboul-Ela2. Prognosis of spinal infections managed by minimal debridement: A case series in two tertiary centers. 02-Mar-2021;12:83
How to cite this URL: Ahmed Ali Mohamed1, Hussein Mohammed Soffar2, Hazem Hassan El Zayat1, Hashem Mohamed Aboul-Ela2. Prognosis of spinal infections managed by minimal debridement: A case series in two tertiary centers. 02-Mar-2021;12:83. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=10616
Abstract
Background: Spinal infections can be challenging in their management and include spondylitis, epidural abscess, and spondylodiscitis. Usual treatment is conservative through antimicrobials or surgery to decompress neural tissue, debride all infected tissues, and fix if needed. We propose the concept of surgery without formal debridement aiming at neural protection.
Methods: The study was performed at two tertiary centers on 25 patients with clinical findings. One patient was treated conservatively and the rest surgically by laminectomy and fixation if needed. Evacuation of fluid pus was performed. In the cervical and the thoracic region, if the granulation tissue was anterior to the cord, only decompression by laminectomy was done.
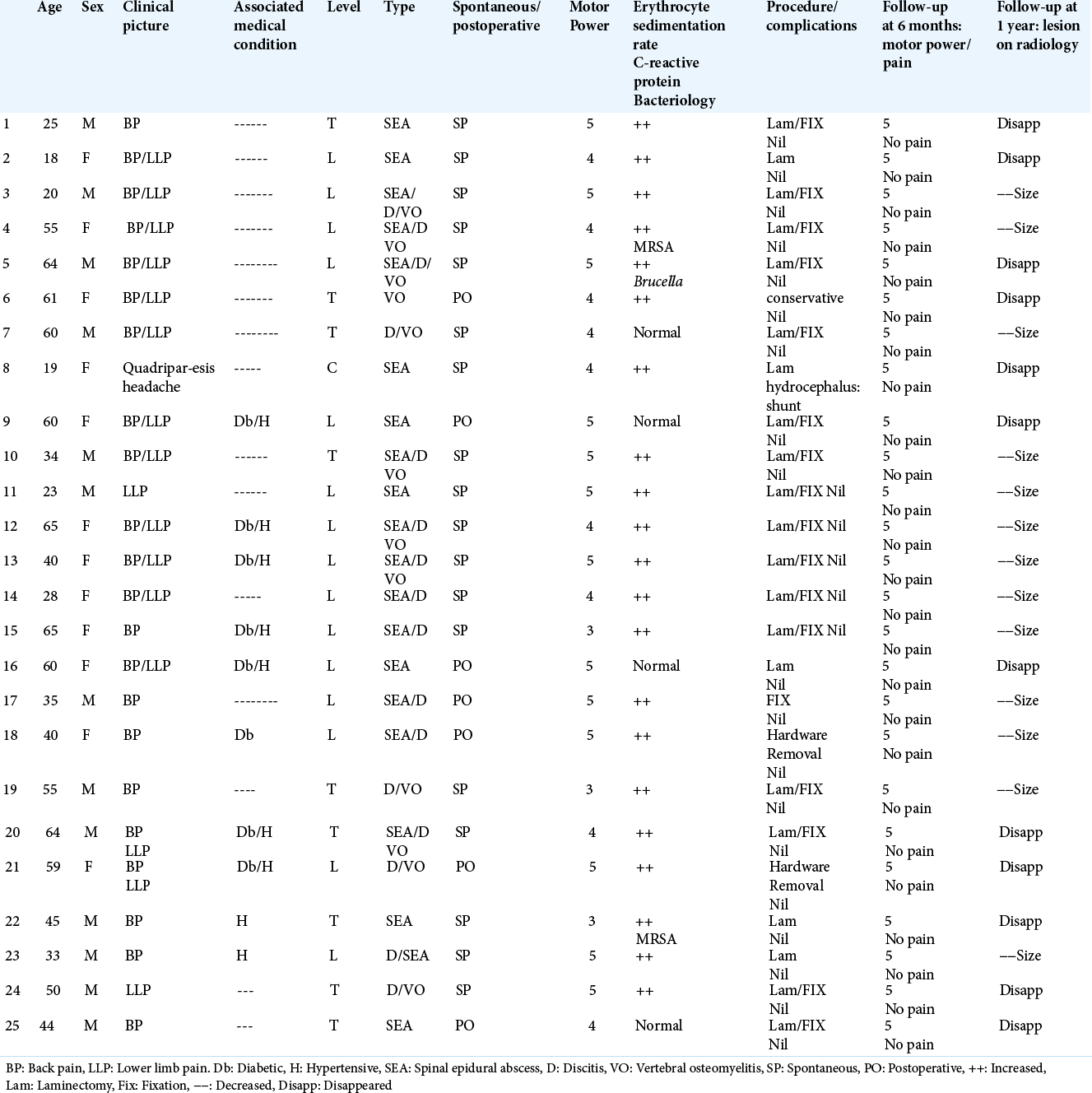
Results: Low back pain was present in 22 cases (88%), 16 cases (64%) had lower limb pain, and 12 cases (48%) had weakness. The level of spinal infection was lumbar in 15 cases (60%), thoracic in 9 cases (36%) cases, and cervical in 1 case (4%). The type of infection was epidural abscess in 20 cases (80%), discitis in 16 cases (64%), and vertebral osteomyelitis in 12 cases (48%). Laminectomy was performed in 20 cases (80%) and fixation in 17 cases (68%). The symptoms improved in all cases. On follow-up, the lesion was reduced in 14 patients (56%) and disappeared in 11 cases (44%). One case required ventriculoperitoneal shunt placement due to postinfectious hydrocephalus.
Conclusion: Dealing with spinal infections surgically through decompression or fixation with minimal debridement of infected tissue appears to be a safe and effective method of management.
Keywords: Debridement, Infection, Spine
INTRODUCTION
Spinal infections represent a grave condition in adults which can be difficult to treat. Most of these patients have a weakened immunity due to comorbidities and malnutrition.[
MATERIALS AND METHODS
After approval from the Institutional Review Board, we retrospectively analyzed patients with spinal infections (discitis, vertebral osteomyelitis, and spinal epidural abscess) managed either surgically or conservatively in Kasr ElAini and Beni-Suef University hospitals during the period between January 2018 and April 2020. The study included patients with clinical manifestations. Patients with normal motor power without back or limb pain and the imaging showing stable spine were excluded from the study. Patients with motor power grade zero for more than 24 h were also excluded from the study. All patients were investigated through imaging tools including plain X-rays, computed tomography scans, or magnetic resonance imaging.
Conservative treatment was considered first in patients with minimal or no neurological deficits. All patients underwent antibiotic therapy for 6–8 weeks with immobilization. Surgery was decided in case of the following: spinal instability, neurological deficits, sepsis unresponsive to antibiotics, and a lesion inaccessible to needle biopsy. The presence of epidural abscess warranted surgery. Spinal fixation was performed if there was apparent spinal instability from the preoperative dynamic X-rays or Kyphotic deformity.
The patients were prepared for surgery by laboratory tests: complete blood picture, coagulation profile, liver, and kidney function tests. Preoperative consent was taken. The prone position was used. After subperiosteal muscle separation, fixation if needed was achieved by transpedicular screws and rods. Laminectomy was then performed for evacuation of the fluid pus in addition to foraminotomy. If the epidural collection was granulation tissue, biopsy was taken in the lumbar (L) region. However, in the cervical and the thoracic (T) region, if the tissue was anterior to the cord, only decompression by laminectomy was done. Closure in layers was performed with subfascial drainage.
Main goals of treatment were clinical improvement of back or limb pain and motor power with normalization of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and blood culture. The patients were followed up with visits after 6 months and 1 year.
RESULTS
The study was conducted on 25 patients. The mean age was 44.8 years old. There were 13 male patients (52%) and 12 female patients (48%).
Low back pain was present in 22 cases (88%), 16 cases (64%) had lower limb pain (sciatic and claudication pain), and 12 cases (48%) had weakness. Eight patients (32%) were diabetic, and 9 cases (36%) were hypertensive. The site of involvement with spinal infection was lumbar spine in 15 cases (60%), thoracic spine in 9 cases (36%) cases, and cervical spine in 1 case (4%). The type of spinal infection was spinal epidural abscess in 20 cases (80%), discitis in 16 cases (64%), and vertebral osteomyelitis in 12 cases (48%). The mode of infection was spontaneous in 18 cases (72%) and postoperative in 7 cases (28%). The infection laboratory indices as ESR, CRP, and blood culture were normal in 4 cases (16%) and abnormal in 21 cases (84 %) [
Surgery was done in the form of laminectomy in 20 cases (80%), and fixation was required in 17 cases (68%). Conservative medical treatment was completed in 1 case (4%) without surgical intervention. The lower limb and low back pain improved in all cases. On follow-up, the lesion decreased in size in 14 patients (56%) and disappeared in 11 cases (44%). One case required ventriculoperitoneal shunt placement due to postinfectious hydrocephalus. Screws removal was performed in two cases of postoperative infections (8%).
DISCUSSION
Bacterial spinal infections are usually caused by pyogenic bacteria, for example, Staphylococcus aureus. They comprise spondylitis, epidural abscess, and spondylodiscitis. In many cases, conservative management with antibiotics is carried out for several weeks up to months and external immobilization using a thoracolumbosacral orthosis.[
The application of instrumentation in the presence of spinal infection is controversial.[
The ideal approach for the treatment of spine infections is yet to be determined. In spine infections, usually, the anterior vertebral elements are involved, which make the anterior surgical approach the most direct route to the infected lesion. However, the posterior approach is preferable as it leads to earlier ambulation, faster rehabilitation, and higher fusion rates.[
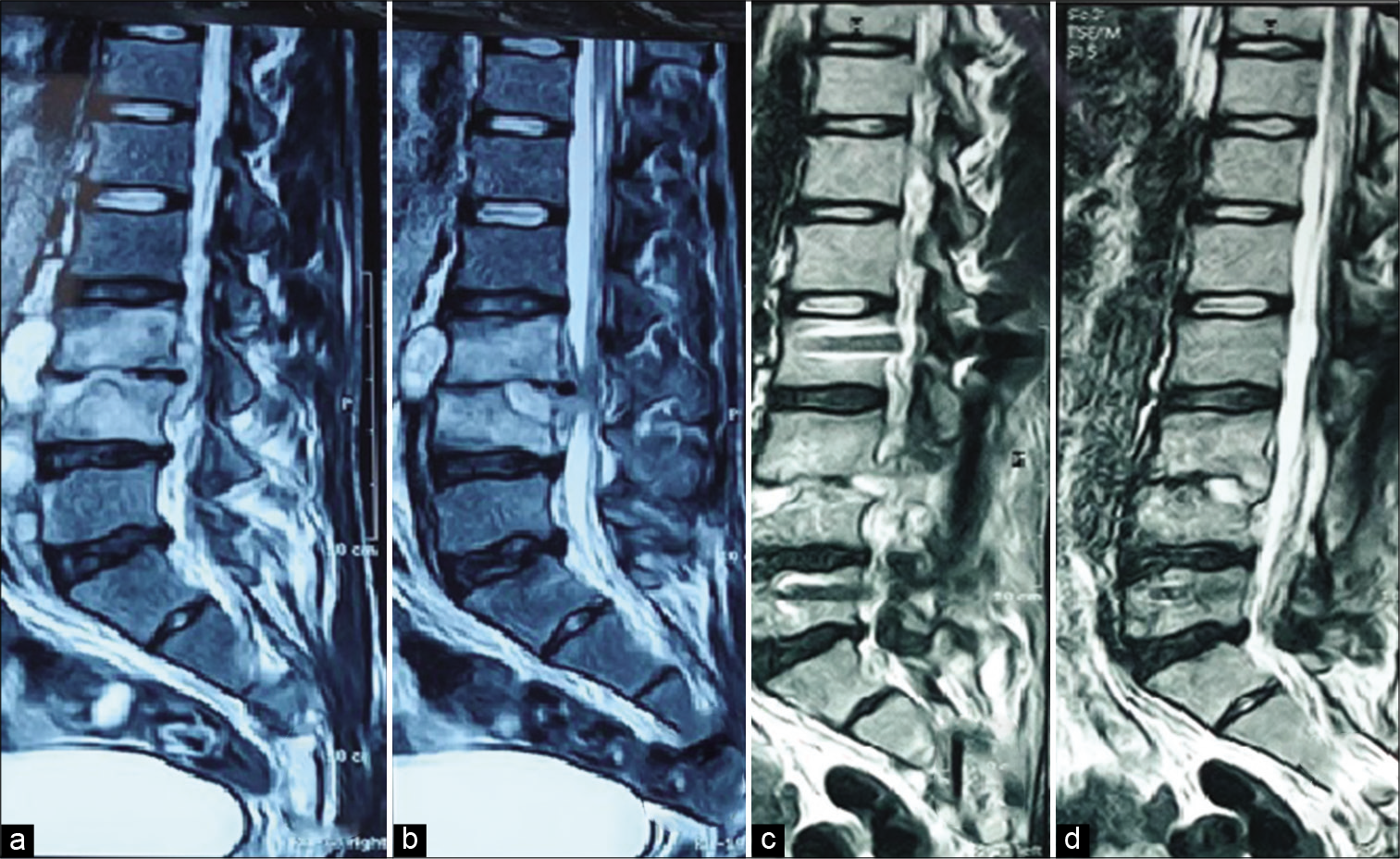
Figure 1:
(a and b) Preoperative magnetic resonance imaging (MRI) sagittal images (c and d) 6 months postoperative images. A 20-year-old male patient presenting with severe low back pain and lower limb claudication pain. MRI showed L3–4 spondylodiscitis, which was operated on by fixation of L2 to L5 and laminectomy of L3 and L5 with sampling of the abscess. The symptoms markedly improved. Culture from the pus was negative. Empirical IV antibiotics were administered for 8 weeks. Serial erythrocyte sedimentation rate and C-reactive protein were eventually normalized.
The prognosis in our series was favorable. In our series, there was only one complication of hydrocephalus, which could rather be considered a comorbidity. It is impossible to compare the rate of complications with other case series due to the small number of cases. There are some limitations of this study. We did not have a control group with formal debridement. We hope that this approach with further larger studies can be validated.
CONCLUSION
Posterior spinal fixation and decompression without excessive debridement, when coupled with thorough antibiotic therapy, are a valuable method of managing spinal infection in patients of such a difficult disease with preexisting comorbidities.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Aljawadi A, Sethi G, Imo E, Arnall F, Choudhry MN, George KJ. Medium-term outcome of posterior surgery in the treatment of non-tuberculous bacterial spinal infection. J Orthop. 2019. 16: 569-75
2. Carragee EJ. Instrumentation of the infected and unstable spine. J Spinal Disord. 1997. 10: 317-24
3. Deininger MH, Unfried MI, Vougioukas VI, Hubbe U. Minimally invasive dorsal percutaneous spondylodesis for the treatment of adult pyogenic spondylodiscitis. Acta Neurochir (Wien). 2009. 151: 1451-7
4. Dobran M, Iacoangeli M, Nasi D, Nocchi N, Di Rienzo A, di Somma L. Posterior titanium screw fixation without debridement of infected tissue for the treatment of thoracolumbar spontaneous pyogenic spondylodiscitis. Asian Spine J. 2016. 10: 465
5. Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Jt Surg. 1983. 65: 19-29
6. Eysel P, Hopf C, Vogel I, Rompe JD. Primary stable anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis?. Eur Spine J. 1997. 6: 152-7
7. Faraj AA, Webb J. Spinal instrumentation for primary pyogenic infection report of 31 patients. Acta Orthop Belg. 2000. 66: 242-7
8. Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000. 25: 1668-79
9. Hee HT, Majd ME, Holt RT, Pienkowski D. Better treatment of vertebral osteomyelitis using posterior stabilization and titanium mesh cages. J Spinal Disord Tech. 2002. 15: 149-56
10. Lee JS, Moon KP, Kim SJ, Suh KT. Posterior lumbar interbody fusion and posterior instrumentation in the surgical management of lumbar tuberculous spondylitis. J Bone Joint Surg Br. 2007. 89B: 210-4
11. Lee MC, Wang MY, Fessler RG, Liauw J, Kim DH. Instrumentation in patients with spinal infection. Neurosurg Focus. 2004. 17: 1-6
12. Lu DC, Wang V, Chou D. The use of allograft or autograft and expandable titanium cages for the treatment of vertebral osteomyelitis. Neurosurgery. 2009. 64: 122-30
13. McGuire RA, Eismont FJ. The fate of autogenous bone graft in surgically treated pyogenic vertebral osteomyelitis. J Spinal Disord. 1994. 7: 206-15
14. McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 cleveland-area hospitals. Clin Infect Dis. 2002. 34: 1342-50
15. Mohamed AS, Yoo J, Hart R, Ragel BT, Hiratzka J, Hamilton DK. Posterior fixation without debridement for vertebral body osteomyelitis and discitis. Neurosurg Focus. 2014. 37: E6
16. Patzakis MJ, Wilkins J, Wiss DA. Infection following intramedullary nailing of long bones. Clin Orthop Relat Res. 1986. 212: 182-91
17. Pee YH, Park JD, Choi YG, Lee SH. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: Autologous iliac bone strut versus cage. J Neurosurg Spine. 2008. 8: 405-12
18. Quiñones-Hinojosa A, Jun P, Jacobs R, Rosenberg WS, Weinstein PR. General principles in the medical and surgical management of spinal infections: A multidisciplinary approach. Neurosurg Focus. 2004. 17: E1
19. Safran O, Rand N, Kaplan L, Sagiv S, Floman Y. Sequential or simultaneous, same-day anterior decompression and posterior stabilization in the management of vertebral osteomyelitis of the lumbar spine. Spine (Phila Pa 1976). 1998. 23: 1885-90
20. Sancineto CF, Barla JD. Treatment of long bone osteomyelitis with a mechanically stable intramedullar antibiotic dispenser: Nineteen consecutive cases with a minimum of 12 months follow-up. J Trauma Inj Infect Crit Care. 2008. 65: 1416-20
21. Sapico FL, Montgomerie JZ. Pyogenic vertebral osteomyelitis: Report of nine cases and review of the literature. Rev Infect Dis. 1979. 1: 754-76
22. Sofianos D, Patel AA. Vertebral osteomyelitis. Contemp Spine Surg. 2010. 11: 1-8
23. Sundararaj GD, Babu N, Amritanand R, Venkatesh K, Nithyananth M, Cherian VM. Treatment of haematogenous pyogenic vertebral osteomyelitis by single-stage anterior debridement, grafting of the defect and posterior instrumentation. J Bone Joint Surg Br. 2007. 89B: 1201-5
24. Sundararaj GD, Amritanand R, Venkatesh K, Arockiaraj J. The use of titanium mesh cages in the reconstruction of anterior column defects in active spinal infections: Can We rest the crest?. Asian Spine J. 2011. 5: 155
25. Zimmerli W. Vertebral osteomyelitis. N Engl J Med. 2010. 362: 1022-9