- School of Medicine, Texas Tech University Health Sciences Center, Lubbock, Texas, United States
- Department of Neurosurgery, University of Oklahoma, Oklahoma City, Oklahoma, United States
- Department of Mathematics, University of Texas Permian Basin, Odessa, United States
- Department of Pediatrics, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
Correspondence Address:
Ryan D. Morgan, Texas Tech University Health Sciences Center, Lubbock, Texas, United States.
DOI:10.25259/SNI_988_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryan D. Morgan1, Abdurrahman F. Kharbat2, Brandon W. Youssi1, John Garza3, Laszlo Nagy4. Prognostic and morphological factors in pediatric cerebellar contusions. 05-Apr-2024;15:117
How to cite this URL: Ryan D. Morgan1, Abdurrahman F. Kharbat2, Brandon W. Youssi1, John Garza3, Laszlo Nagy4. Prognostic and morphological factors in pediatric cerebellar contusions. 05-Apr-2024;15:117. Available from: https://surgicalneurologyint.com/surgicalint-articles/12846/
Abstract
Background: Although uncommon, cerebellar contusions are associated with significant morbidity and mortality. Literature is lacking in the prognostic and morphological factors relating to their clinical picture and outcomes, especially within children. The objective of this study is to evaluate prognostic and anatomic factors in the clinical picture of cerebellar contusions, including effacement of the 4th ventricle and cisterna magna.
Methods: This is a retrospective chart review over 11 years across two medical centers. Patients included were under 18 years who presented with a cerebellar contusion. Patients were stratified within the study group based on discharge Glasgow outcome scale (GOS) and reviewed for prognostic factors contributing to outcome. Mid sagittal area of the 4th ventricle and cisterna magna were measured using magnetic resonance imaging and compared within the groups.
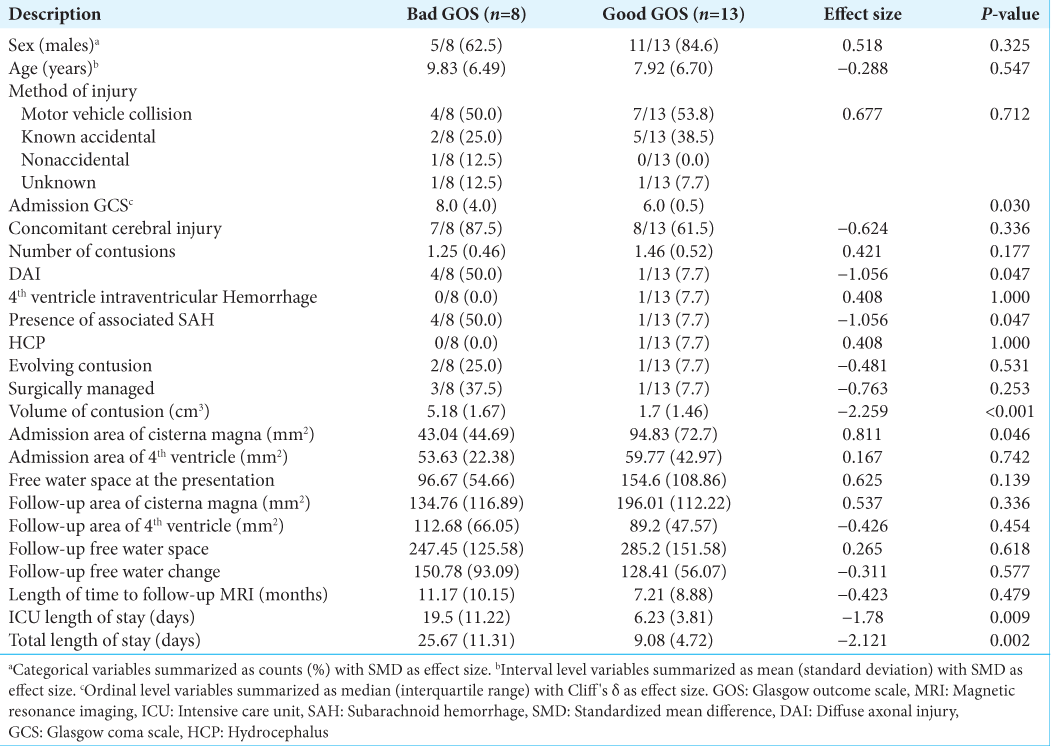
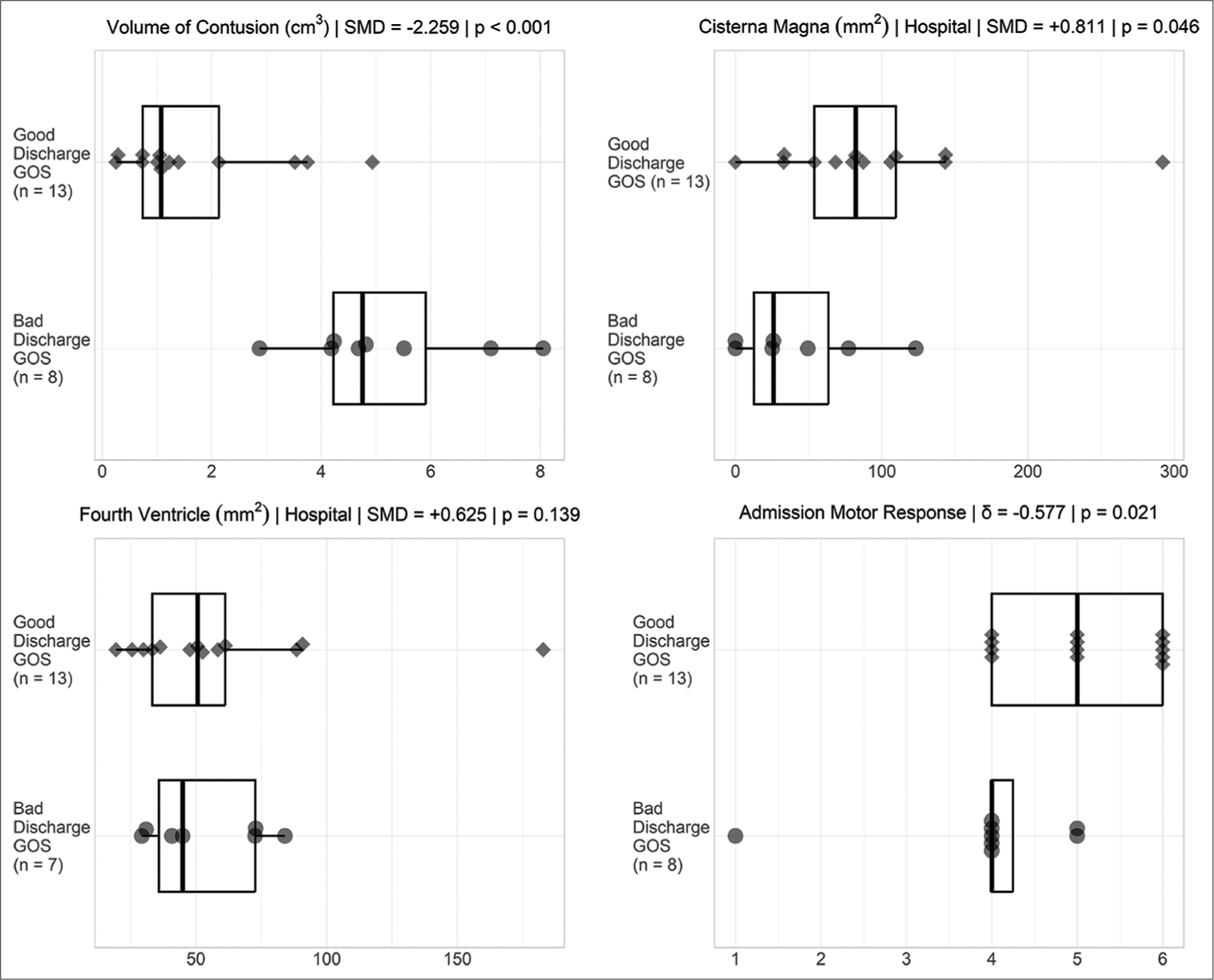
Results: A total of 21 patients met the study criteria, of which 16 (76.2%) were male, with an average patient age of 8.65 years. Poor outcome at discharge (GOS P = 0.003), admission motor response (P = 0.006), pupil reactivity (P = 0.014), presence of concomitant subarachnoid hemorrhage (P = 0.010), contusion volume (P P = 0.012). Patients with poor outcomes were also more likely to require surgical intervention (P = 0.042).
Conclusion: There are multiple prognostic factors associated with the overall outcome following cerebellar contusions. The rate of good outcomes in this study was superior to that in previous studies in adults.
Keywords: Cerebellar contusions, Intraparenchymal hemorrhage, Pediatrics, Posterior fossa, Traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) represents the leading cause of pediatric death and disability annually, with estimates of 70–75 hospitalizations/100,000.[
Due to the low incidence of cerebellar contusions, few studies have endeavored to establish prognostic factors in patients sustaining cerebellar contusions. These studies have historically generated small sample sizes with limited statistical power.[
MATERIALS AND METHODS
Study population
Following formal approval from the Institutional Review Board, we performed a retrospective analysis of patients who presented to the emergency department (ED) with cerebellar contusions from the years 2012 to 2022. This study was performed at two medical centers, one of which is an academic institution with a level 1 trauma center that acts as a tertiary care facility and the other with a level 2 trauma center. Patients were included in the study if they were younger than 18 years of age on sustaining cerebellar contusion(s). To gather the list of patients meeting study criteria, the IT departments were asked to run a list of all patients who presented during these years with a TBI and/or basilar skull fractures. Of the lists provided, we manually screened for all patients with cerebellar contusions based on magnetic resonance imaging (MRI) imaging. Patients were excluded if they had incomplete or missing data.
Data acquisition
Due to the retrospective nature of this study, all data were gathered through electronic medical records, including physician notes, nurse notes, operative notes, and imaging studies. Demographic data obtained included patient age, sex, and mechanism of injury. Further, data were obtained regarding patients’ presentation status, including admission GCS, motor response, pupil reactivity, and symptoms at presentation. Subsequently, related data were obtained from imaging studies, including the type of contusions, number of contusions present, associated diffuse axonal injury, concomitant cerebral injury, location of contusion (central vs hemispheric), concomitant SAH, the presence of hydrocephalus, intraventricular hemorrhage, and whether the contusion progressively increased in size. Data were collected on whether surgical operations were warranted and the method used for surgical evacuation.
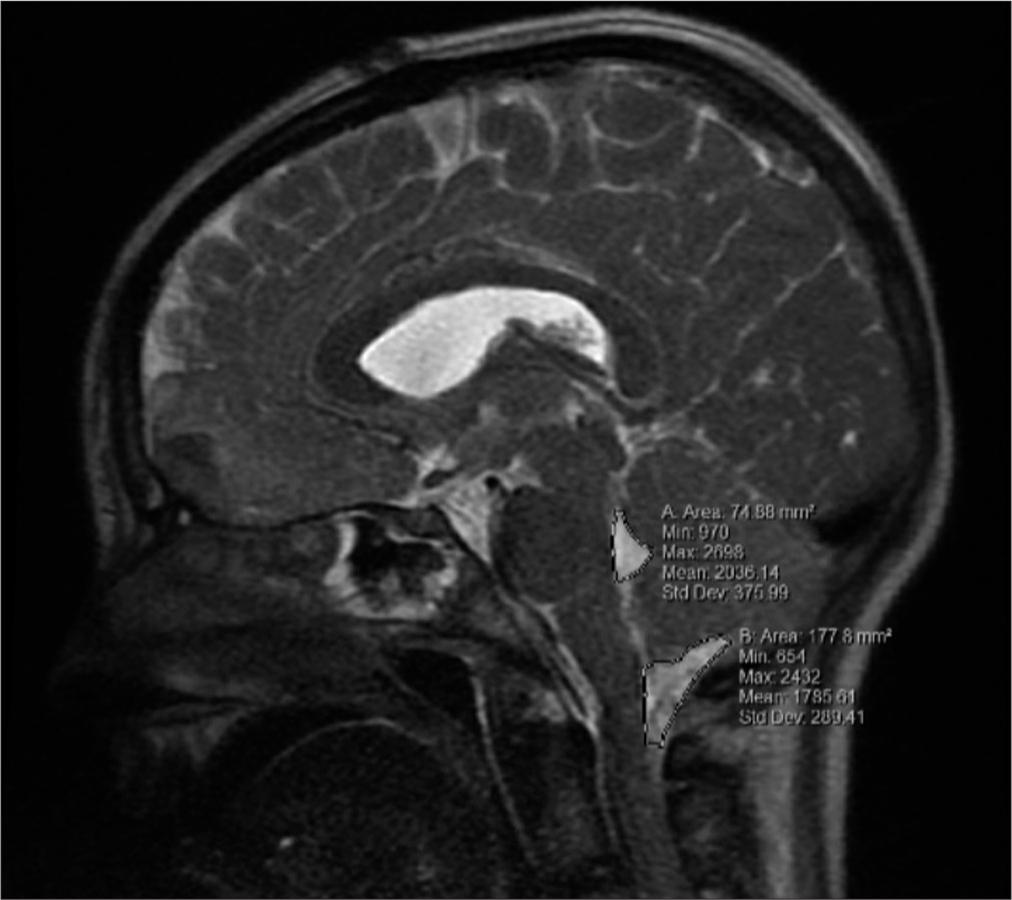
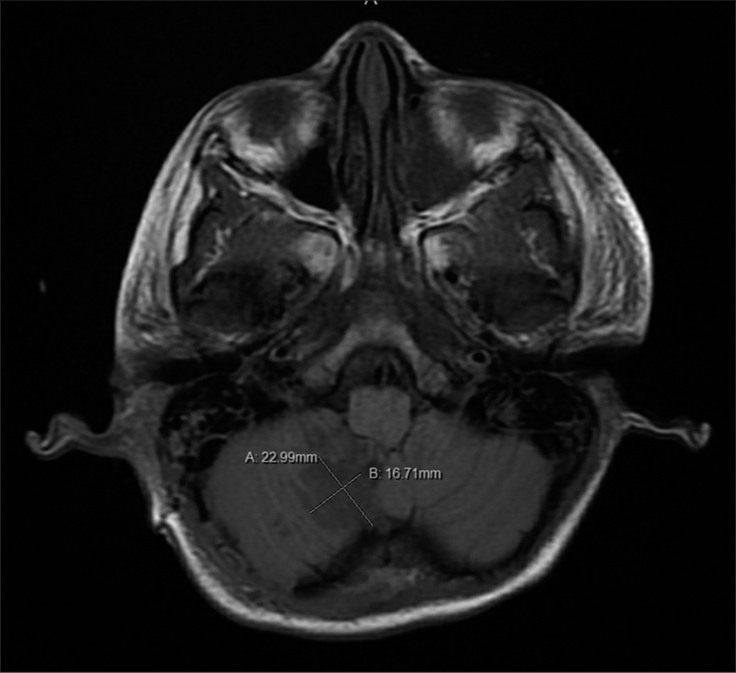
In addition to the data mentioned above collected, measurements were also taken of the 4th ventricular and cisterna Magna’s mid-sagittal area, and the volume of the contusions. All measurements were obtained thrice using MRI under the supervision of the attending neurosurgeon. Initial MRI imaging was obtained around the 24 h mark with no MRI being obtained later than the 36-h mark. The 4th ventricular and cisterna Magna’s areas were measured in the mid-sagittal plane utilizing the area measuring tool within the imaging program [
The primary outcome of the study was the discharge Glasgow outcome scale (GOS). A GOS of 4 or 5 was considered a good outcome, while a GOS of 1, 2, or 3 was considered a poor or bad outcome. Secondary outcome measures included total length of stay (LOS), intensive care unit (ICU) LOS, and follow-up GOS, which was obtained from follow-up neurosurgical clinic notes.
Statistical analysis
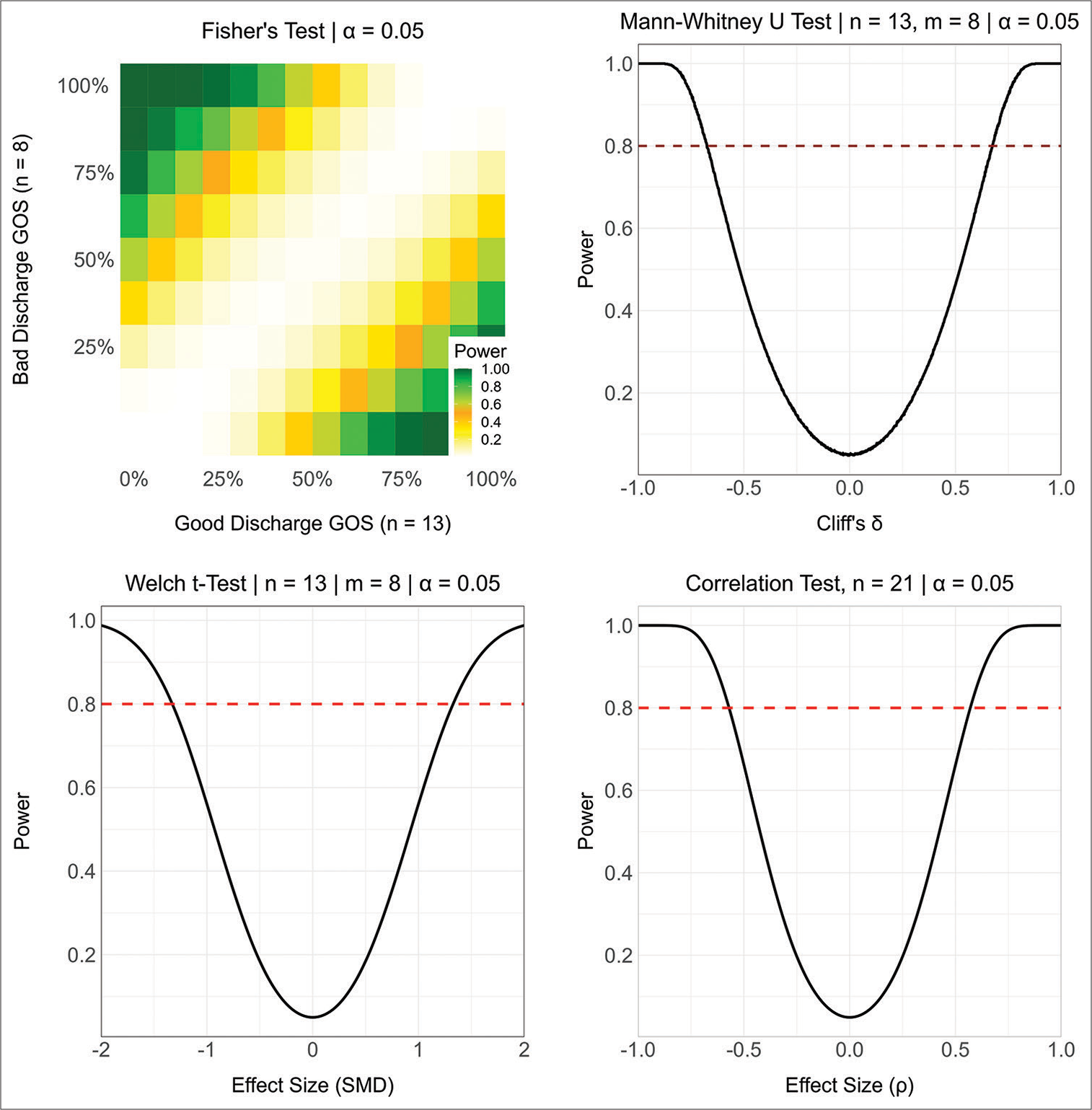
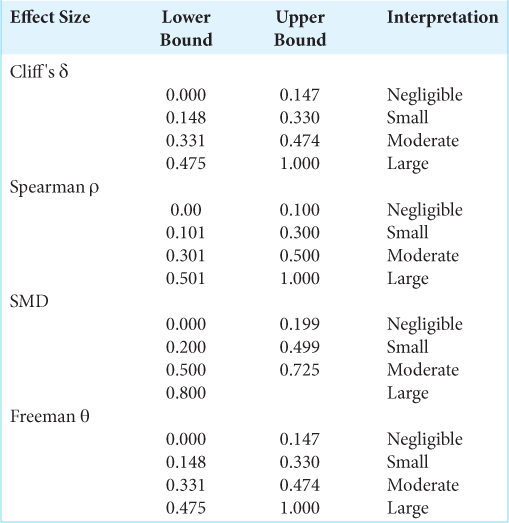
Statistical analyses are based on inferential statistics with an emphasis on hypothesis testing using a significance level of α = 0.05, two-sided P-values, and independent samples. Analyses included partitioning subjects into two independent groups and then comparing all other variables across groups. Binary and multilevel nominal variables are summarized using counts and percentages with differences across groups tested using Fisher’s test and the standardized mean difference (SMD) as effect size. Ordinal level variables are summarized using the median and interquartile range (IQR) with differences across groups testing using the Mann–Whitney U-test with Cliff’s δ as effect size. Interval level variables are summarized as mean and standard deviation (SD) with differences across groups tested using the permutational unequal variance t-test based on 1000 randomizations and using the SMD as effect size. Associations between interval and ordinal level variables are tested using the Spearman correlation, and associations between binary and ordinal variables are tested using the exact Cochrane-Armitage test of trend with Freeman’s θ as effect size. Hypothesis tests and descriptive statistics exclude any missing values. To simplify the presentation and interpretation of results, no adjustments to P-values to control the false discovery rate or the family-wise error rate have been made, and no multivariate analyses have been used. This project does not involve parameter estimation, so that confidence intervals for effect sizes and population parameters are not provided. The sample sizes were determined based on data availability and exclusion criteria so that no a priori power analyses were conducted. To aid in the interpretation of non-significant P-values, Supplementary
RESULTS
Demographics
A total of 4238 patient ED records were searched and 27 patients presented during the period with cerebellar contusions. Of these patients, 21 were included in the study, as the others did not meet the inclusion criteria. The mean age was 8.65 (SD = 6.53), and 76.2% (n = 16) were male. The most common reason for the presentation was a motor vehicle collision (MVC) (52.4%), followed by a fall from height/blow to the head (33.3%), gunshot wound-associated injury (9.5%), and nonaccidental trauma (4.8%). Injuries from the gunshot wound were not caused by direct trauma from a penetrating injury. The median GCS on admission was 7.0 (IQR 4.0 [
Predictors of outcome
Of the 21 patients with cerebellar contusions, 13 had a good outcome at discharge. Patients with bad outcomes at discharge more commonly presented with lower GCS (P = 0.030), lower admission motor response (P = 0.021), worse pupil reactivity (P = 0.029), presence of concomitant SAH (P = 0.047), larger contusion volume (P < 0.001), and diminished area of the cisterna magna (P = 0.046) [
DISCUSSION
Compared to intracerebral contusions, cerebellar contusions are less common and have previously reported incidence rates of <1.0%, with ranges between 0.4% and 0.8%.[
Previous studies have explored the predictors of outcome in patients following cerebellar contusions in adults.[
The location of the hematoma has previously been shown to be related to the outcome following cerebellar contusions. Contusions have been considered either peripheral or central, with the latter being correlated to worse outcomes. This is thought to be due to the location of the deep cerebellar nuclei.[
There is a broad range of symptoms that patients have previously presented with following cerebellar contusions. These can be classified as symptoms of cerebellar dysfunction or symptoms of increased intracranial pressure (ICP). Cerebellar dysfunctions include dysdiadochokinesis, nystagmus, hypotonia, ataxia, dysmetria, tremor, and vertigo. Typical signs of high ICP include headache, decreased arousal/altered sensorium, vomiting, and pupil irregularity.[
The historical outcome of patients who have sustained cerebellar contusions is not favorable. Many studies have high mortality rates and a low number of good outcomes following surgery. Mortality rates are typically reported around 40%, whereas poor outcomes are usually reported between 20% and 100%, with an average of 60%.[
Recently, conservative management has become popular in the care of patients with cerebellar contusions. Guidelines suggest that surgery is appropriate when the contusion is >3 cm in diameter or 15 mL in size, compression of cisterna magna or 4th ventricle is present, and/or acute hydrocephalus or associated epidural/subdural hematomas in the posterior fossa are present.[
In the literature regarding cerebellar contusions, few studies and case reports present that compression of the 4th ventricle and effacement of the basal cisterns are correlated with worse outcomes.[
Limitations
A limitation of this study is the retrospective nature, which can induce bias. Furthermore, though the sample size is comparable to existing literature, our study’s power is limited by the small sample size of 21 patients. Another limitation of this study was the time to initial MRI after presentation. All patients presented in this study had an MRI within the first 36 hours of admission; however, a uniform timing of MRI would have been optimal. Due to the nature of TBI, patient presentations were heterogenous, with multiple patients presenting with concomitant cerebral contusions. Finally, only a single patient had an MRI before the injury; therefore, follow-up MRIs were used for comparison.
CONCLUSION
In pediatric patients with cerebellar contusions, the decreased size of their cisterna magna, reduced pupil reactivity, decreased GCS, decreased admission motor response, and concomitant SAH have all shown a negative correlation with discharge outcome. Taken together, these components serve as prognostic factors. Pediatric patients demonstrated a good overall outcome following both surgical and conservative management. Future research is warranted further to explore these associations and their potential clinical applications.
Data availability statement
All data generated or analyzed during this study are included in this article. Further, enquiries can be directed to the corresponding author.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
Patient’s consent are not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTARY
References
1. Bhardwaj S, Sharma V, Sharma S, Purohit D, Chopra S. Traumatic posterior fossa hematoma, a rare entity: Study of 21 cases. J Neurosci Rural Pract. 2019. 10: 675-82
2. Chen C, Peng J, Sribnick EA, Zhu M, Xiang H. Trend of age-adjusted rates of pediatric traumatic brain injury in U.S. emergency departments from 2006 to 2013. Int J Environ Res Public Health. 2018. 15: 1171
3. Cooper PR. Delayed traumatic intracerebral hemorrhage. Neurosurg Clin N Am. 1992. 3: 659-65
4. D’Avella D, Cacciola F, Angileri FF, Cardali S, La Rosa G, Germano A. Traumatic intracerebellar hemorrhagic contusions and hematomas. J Neurosurg Sci. 2001. 45: 29-37
5. D’Avella D, Servadei F, Scerrati M, Tomei G, Brambilla G, Angileri FF. Traumatic intracerebellar hemorrhage: Clinicoradiological analysis of 81 patients. Neurosurgery. 2002. 50: 16-25 discussion 25-17
6. Harsh V, Prakash A, Barry JM, Kumar A. Traumatic intracerebellar haematoma: To operate or not to operate?. Br J Neurosurg. 2014. 29: 353-7
7. Holland JN, Schmidt AT. Static and dynamic factors promoting resilience following traumatic brain injury: A brief review. Neural Plast. 2015. 2015: 902802
8. Javed G, Bashir S, Islam Y. Traumatic midline cerebellar contusion in 2-year-old male child-case report and review of literature. Egypt J Neurosurg. 2018. 33: 6
9. Karasawa H, Furuya H, Naito H, Sugiyama K, Ueno J, Kin H. Acute hydrocephalus in posterior fossa injury. J Neurosurg. 1997. 86: 629-32
10. Kariyattil R, Rahim MI, Muthukuttiparambil U. Cerebellar mutism following closed head injury in a child. Sultan Qaboos Univ Med J. 2015. 15: e133-5
11. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996. 27: 1304-5
12. Kulshreshtha V, Tripathi P, Jaiswal G, Gupta TK. Traumatic cerebellar hematoma in paediatric patient-a case report and review of literature. Rom Neurosurg. 2016. 30: 566-72
13. Nagata K, Ishikawa T, Ishikawa T, Shigeno T, Kawahara N, Asano T. Delayed traumatic intracerebellar hematoma: Correlation between the location of the hematoma and the pre-existing cerebellar contusion--case report. Neurol Med Chir (Tokyo). 1991. 31: 792-6
14. Pandey N, Singh R, Singh R. Traumatic cerebellar hematoma: A tertiary care experience of 23 conservatively managed cases. Asian J Neurosurg. 2020. 15: 882-8
15. Patnaik A, Mahapatra AK. Traumatic cerebellar haematoma: A review. Indian J Neurotrauma. 2013. 10: 24-9
16. Pollak L, Rabey JM, Gur R, Schiffer J. Indication to surgical management of cerebellar hemorrhage. Clin Neurol Neurosurg. 1998. 100: 99-103
17. Sato K, Hinokuma K, Matsuzawa Y, Takehara S, Uemura K, Ninchoji T. Clinical study of traumatic cerebellar contusion. No Shinkei Geka. 1987. 15: 1285-9
18. Takeuchi S, Takasato Y, Masaoka H, Hayakawa T. Traumatic intra-cerebellar haematoma: Study of 17 cases. Br J Neurosurg. 2010. 25: 62-7
19. Tsai FY, Teal JS, Itabashi HH, Huprich JE, Hieshima GB, Segall HD. Computed tomography of posterior fossa trauma. J Comput Assist Tomogr. 1980. 4: 291-305
20. Zuccarello M, Andrioli GC, Fiore DL, Longatti PL, Pardatscher K, Zampieri P. Traumatic posterior fossa haemorrhage in children. Acta Neurochir (Wien). 1982. 62: 79-85