- Department of Neurosurgery, Hull Royal Infirmary, Yorkshire, United Kingdom.
Correspondence Address:
Duncan Henderson, Department of Neurosurgery, Hull Royal Infirmary, Yorkshire, United Kingdom.
DOI:10.25259/SNI_103_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Duncan Henderson, Arif Zafar, Anna Bjornson, Adam Razak, Shailendra Achawal, Mihai Danciut, Aubrey Smith, Gerry O’Reilly, Chittoor Rajaraman, Anuj Bahl. Prognostic factors following resection of intracranial metastases. 27-May-2022;13:219
How to cite this URL: Duncan Henderson, Arif Zafar, Anna Bjornson, Adam Razak, Shailendra Achawal, Mihai Danciut, Aubrey Smith, Gerry O’Reilly, Chittoor Rajaraman, Anuj Bahl. Prognostic factors following resection of intracranial metastases. 27-May-2022;13:219. Available from: https://surgicalneurologyint.com/surgicalint-articles/11624/
Abstract
Background: The aim of this study was to identify prognostic factors associated with resection of intracranial metastases.
Methods: A retrospective case series including patients who underwent resection of cranial metastases from March 2014 to April 2021 at a single center. This identified 112 patients who underwent 124 resections. The median age was 65 years old (24–84) and the most frequent primary cancers were nonsmall cell lung cancer (56%), breast adenocarcinoma (13%), melanoma (6%), and colorectal adenocarcinoma (6%). Postoperative MRI with contrast was performed within 48 hours in 56% of patients and radiation treatment was administered in 41%. GraphPad Prism 9.2.0 was used for the survival analysis.
Results: At the time of data collection, 23% were still alive with a median follow-up of 1070 days (68–2484). The 30- and 90-day, and 1- and 5-year overall survival rates were 93%, 83%, 35%, and 17%, respectively. The most common causes of death within 90 days were as follows: unknown (32%), systemic or intracranial disease progression (26%), and pneumonia (21%). Age and extent of neurosurgical resection were associated with overall survival (P 70 had a median survival of 5.4 months compared with 9.7, 11.4, and 11.4 for patients
Conclusion: Age and extent of resection are potential predictors of long-term survival.
Keywords: Brain metastasis, Breast cancer, Nonsmall cell lung cancer, Stereotactic radiosurgery, Whole-brain radiotherapy
INTRODUCTION
The incidence of brain metastases is increasing, likely due to the improved systemic therapies resulting in prolonged overall survival[
Craniotomy and resection of a brain metastasis can prolong overall survival in select patients.[
MATERIALS AND METHODS
The aim of this study was to identify prognostic factors associated with resection of intracranial metastases. Before data collection, this study was formally registered with the local audit department, the ethics and methodology of the study were reviewed and signed off by the clinical governance lead. A retrospective study was carried out at a single center, Hull Royal Infirmary. Data collection occurred from May 12, 2021, to July 19, 2021. All patients who underwent attempted neurosurgical resection of a cranial metastasis between March 2014 and April 2021 were included in the study. Clinic letters, radiology and histology reports, and operation notes were used for data collection. One hundred and twenty-six patients were identified. However, 14 patients were excluded from the study and the reasons included; no available information, nonmetastatic histological diagnosis such as glioblastoma or lymphoma, biopsy of metastasis, treated through cyst aspiration, and insertion of Ommaya reservoir. Therefore, 112 patients were included who underwent a total of 124 resections. The diagnosis was concluded from the neurosurgical histological result and imaging available to the neuro-oncology multidisciplinary team. Biopsy results for any systemic lesions targeted were not available for analysis as these procedures would have been performed at a different hospital.
Neurosurgery clinic letters describing referral for consideration of whole-brain radiotherapy (WBRT) or STRS were not adequate to be recorded as having received treatment. The treatment reports stating the radiation dose (Gy) and fractions (WBRT) or number of target lesions (STRS) were reviewed to confirm adjuvant radiation administered. The extent of surgical resection was defined as unclear in patients where MRI was contraindicated, if they were too unwell for appropriate investigations, if no MRI was available, or if unable to distinguish residual from recurrent disease. Gross-total resection included cases where a postcontrast MRI did not demonstrate any residual enhancing material whereas cases were classified as sub-total resection if there was a tiny or small volume of enhancing material at the surgical site. Debulking included cases where there was either a large residual or limited resection performed. The extent of surgical resection was formally reported by a neuroradiologist and reviewed by a neurosurgeon.
Statistical analysis was carried out using GraphPad Prism 9.2.0 and P < 0.05 was deemed statistically significant. For the survival analysis, 1 month was defined as 28 days. Kaplan– Meier graphs were used and the statistical significance was calculated through the Log rank (Mantel–Cox) test.
RESULTS
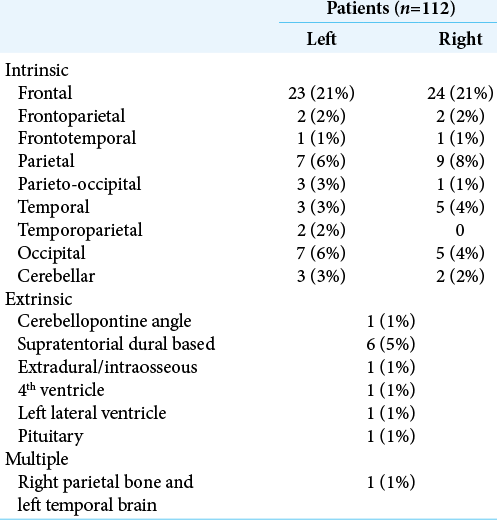
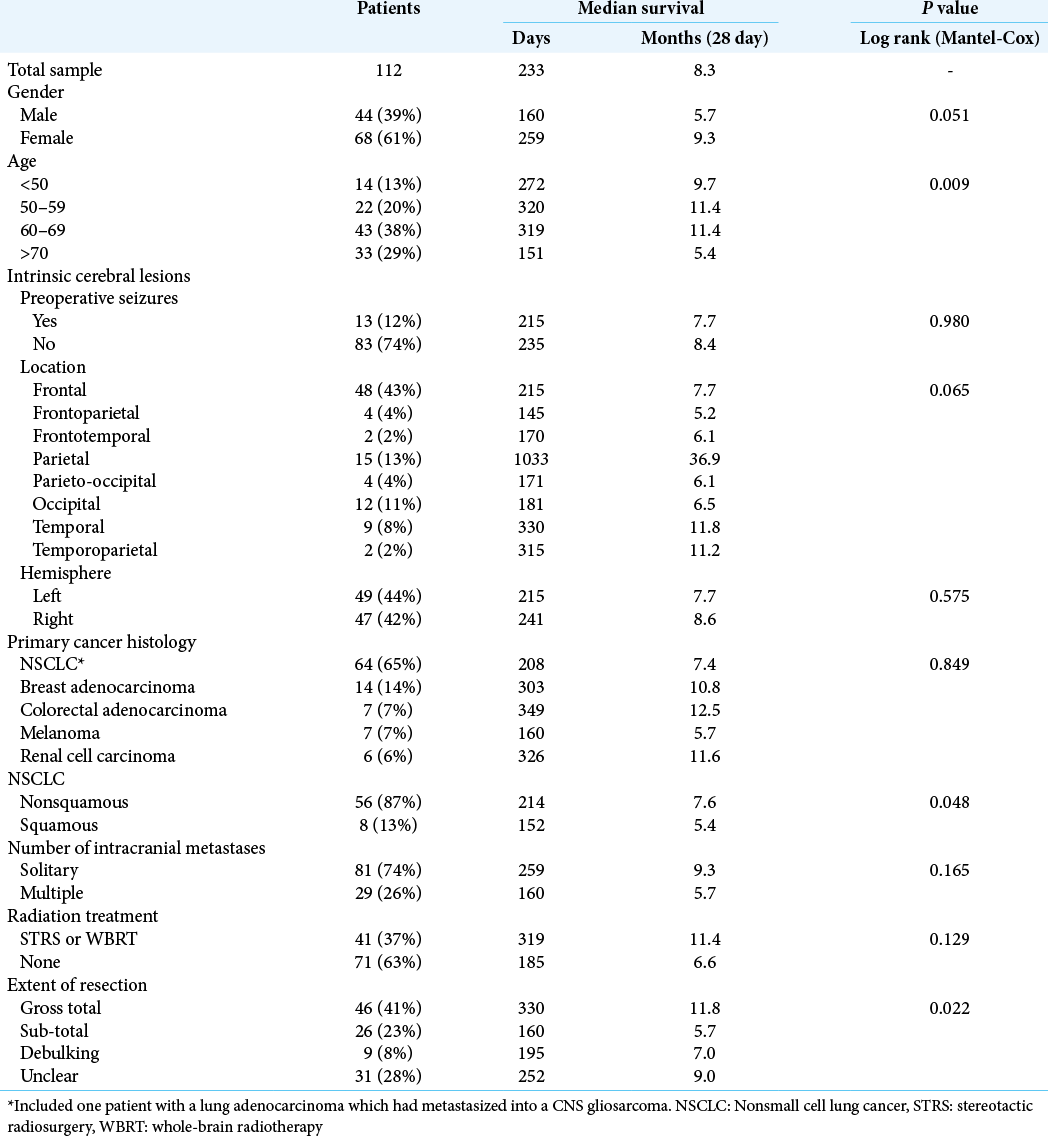
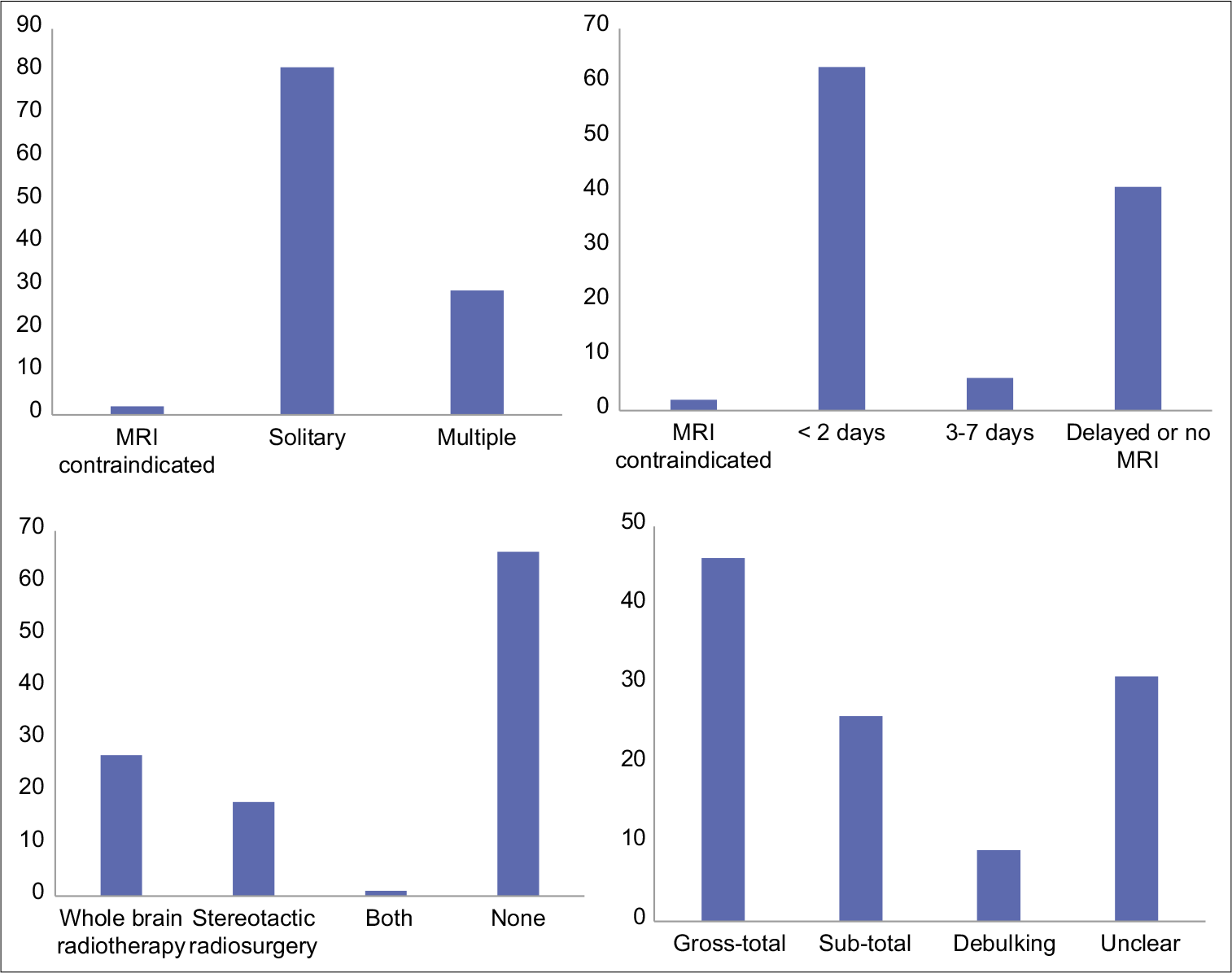
The median age was 65 years old (24 to 84). The male-to-female ratio was 1:1.54 with 44 (39%) males and 68 (61%) females. There were 81 patients (72%) with a solitary cranial metastasis, 29 (26%) with multiple intracranial metastases, and in 2 (2%), MRI was contraindicated with only a preoperative CT available. The metastasis location most frequently involved the frontal lobe (frontal, frontoparietal, and frontotemporal [53 patients, 47%]), [
The primary histological diagnosis was NSCLC in 63 patients (56%) which included one patient with an adenocarcinoma metastasis within a gliosarcoma, breast adenocarcinoma in 14 (12%), melanoma in 7 (6%), colorectal adenocarcinoma in 7 (6%), renal cell carcinoma in 6 (5%), esophageal adenocarcinoma in 4 (4%), endometrial sarcoma in 2 (2%), endometrial adenocarcinoma in 1 (1%), ovarian adenocarcinoma in 2 (2%), bladder transitional cell carcinoma in 1 (1%), anal squamous cell carcinoma in 1 (1%), prostate adenocarcinoma in 1 (1%), ethmoid air sinus adenocarcinoma in 1 (1%), and small cell lung cancer in 2 (2%).
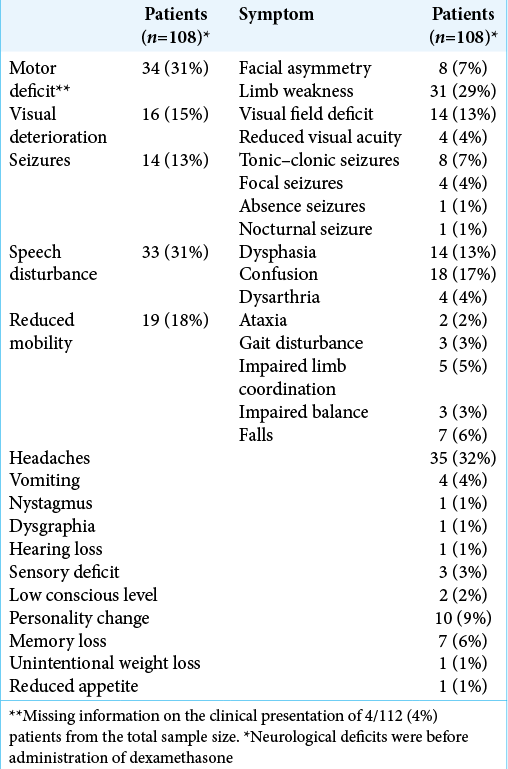
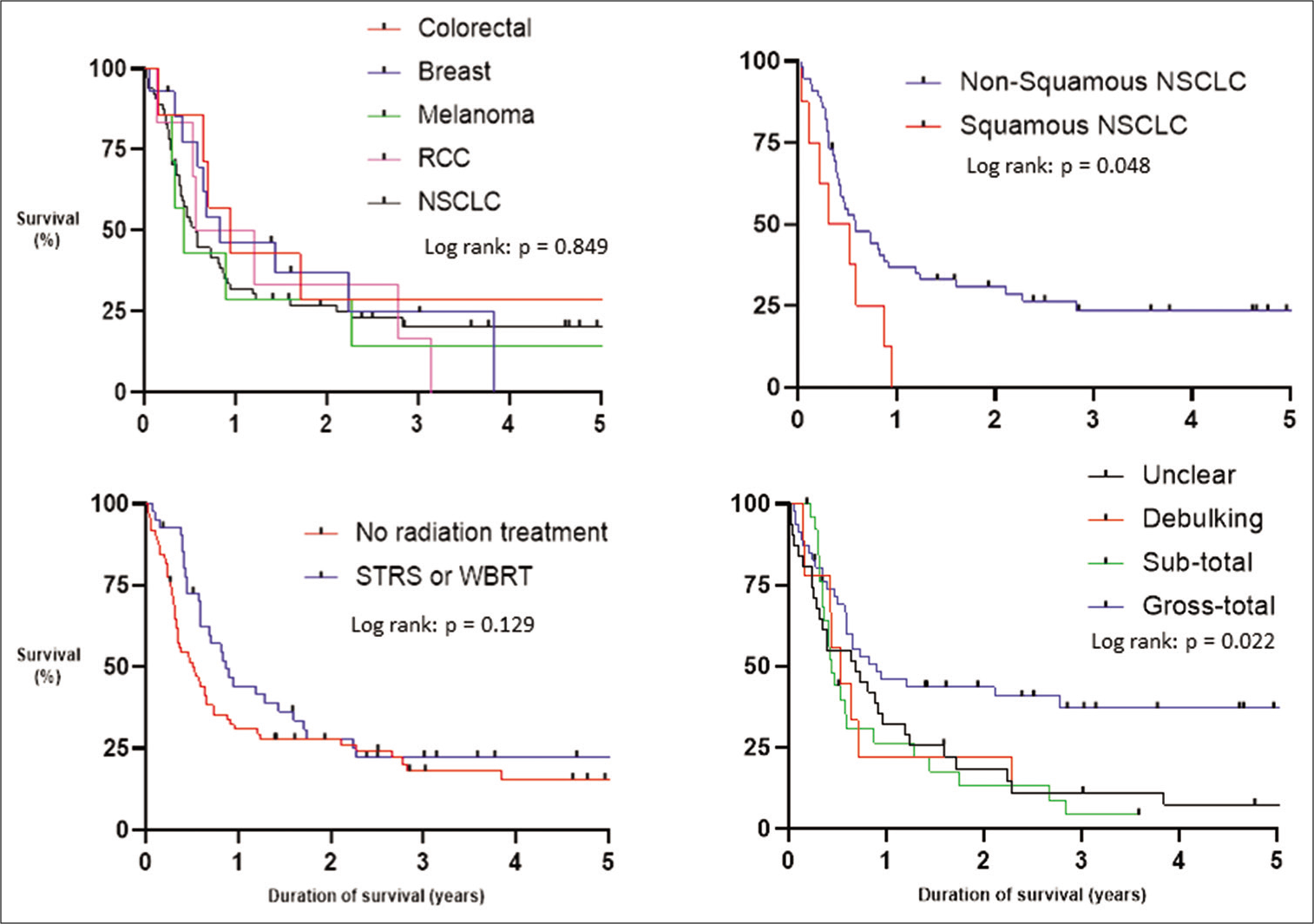
Postoperative MRI with contrast was carried out within 48 hours of surgery in 63 patients (56%), between 3 and 7 days in 6 (5%) and was delayed or not performed at all in 41 patients (37%). MRI scanning was contraindicated in 2 patients (2%). Gross-total resection was achieved in 46 patients (41%), sub-total in 26 (23%), debulking in 9 (8%), and the extent of surgical resection was unclear in 31 patients (28%). Adjuvant STRS to the resection cavity was not carried out. However, 46 (41%) underwent radiation treatment for residual, recurrent, or additional intracranial metastatic disease. This included 27 (24%) who received WBRT, 18 (16%) received STRS, and 1 (1%) who received both WBRT and STRS [
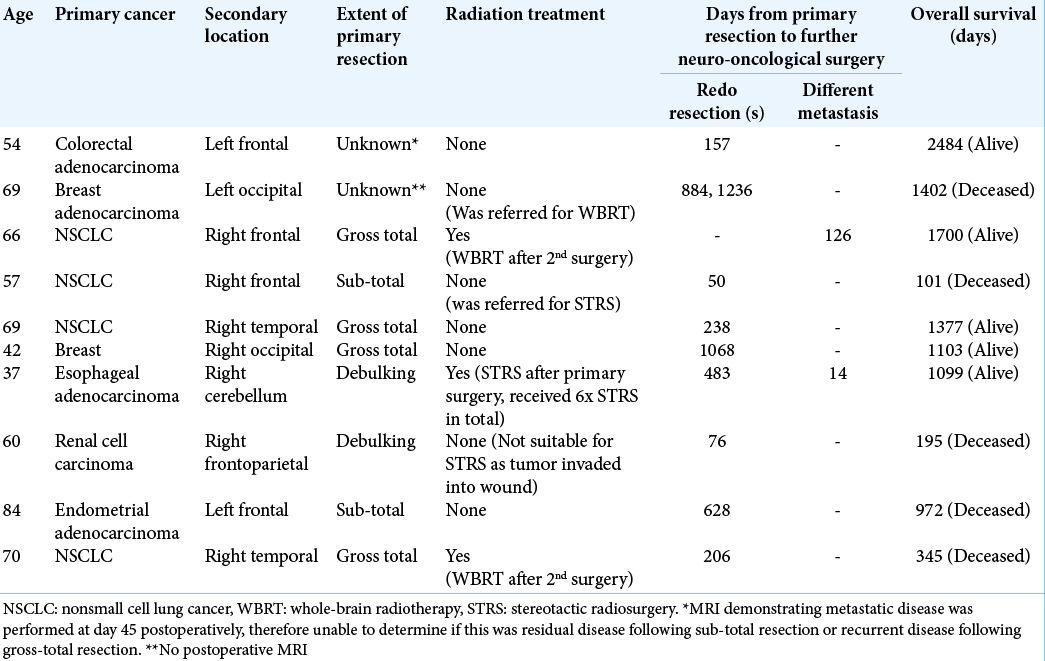
Of the total 112 patients, 10 (9%) underwent further resections of brain metastases, including 10 redo resections for recurrence at the surgical site and two resections of discrete lesions at a different intracranial site [
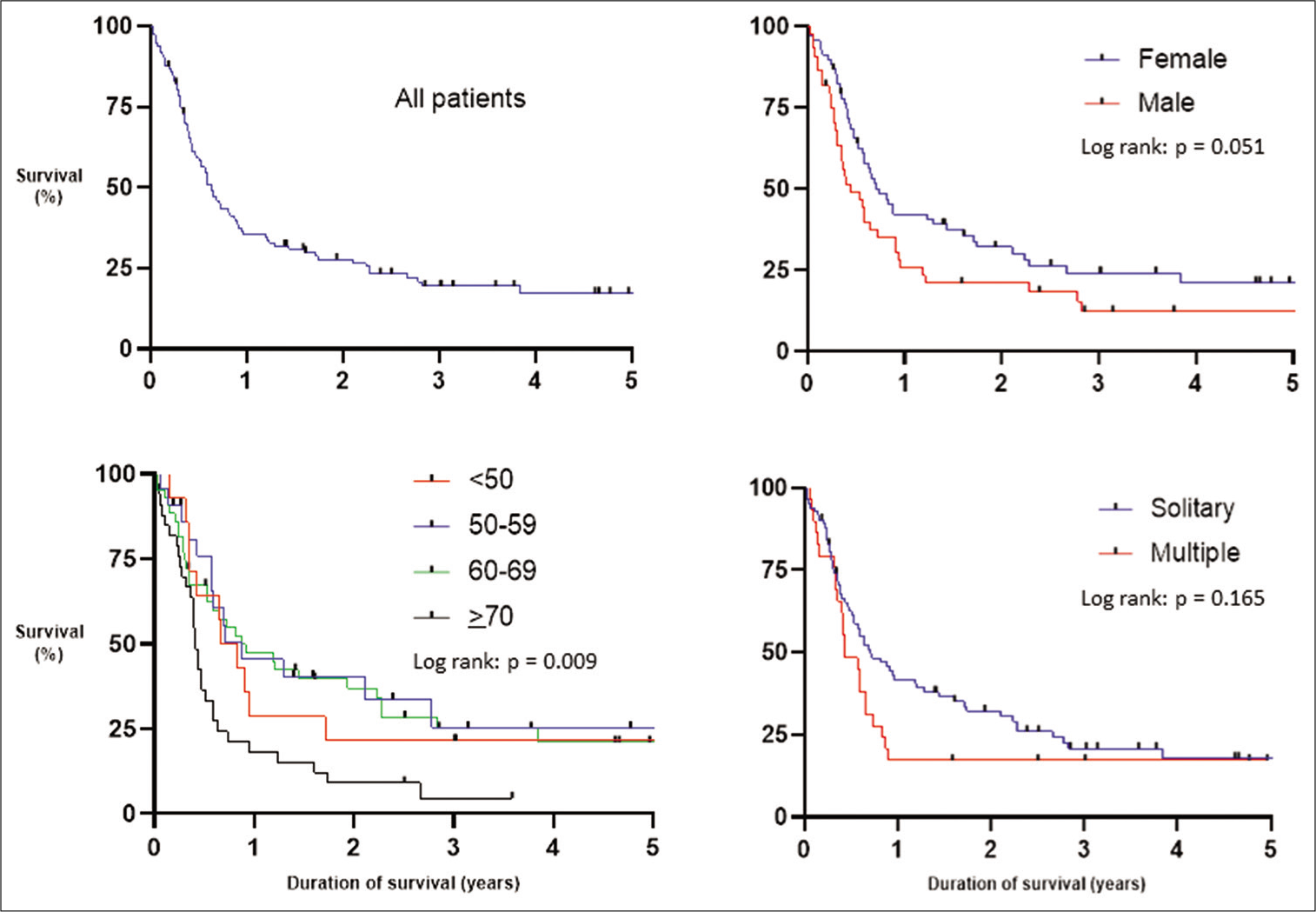
At the time of data collection, 26/112 (23%) were still alive and the median follow-up for those patients was 1070 days (68– 2484). Including the total patient cohort, the rates of overall survival were as follows: 1 month = 93%, 6 months = 58%, 1 year = 35%, and 5 years = 17%, with a median survival of 233 days [
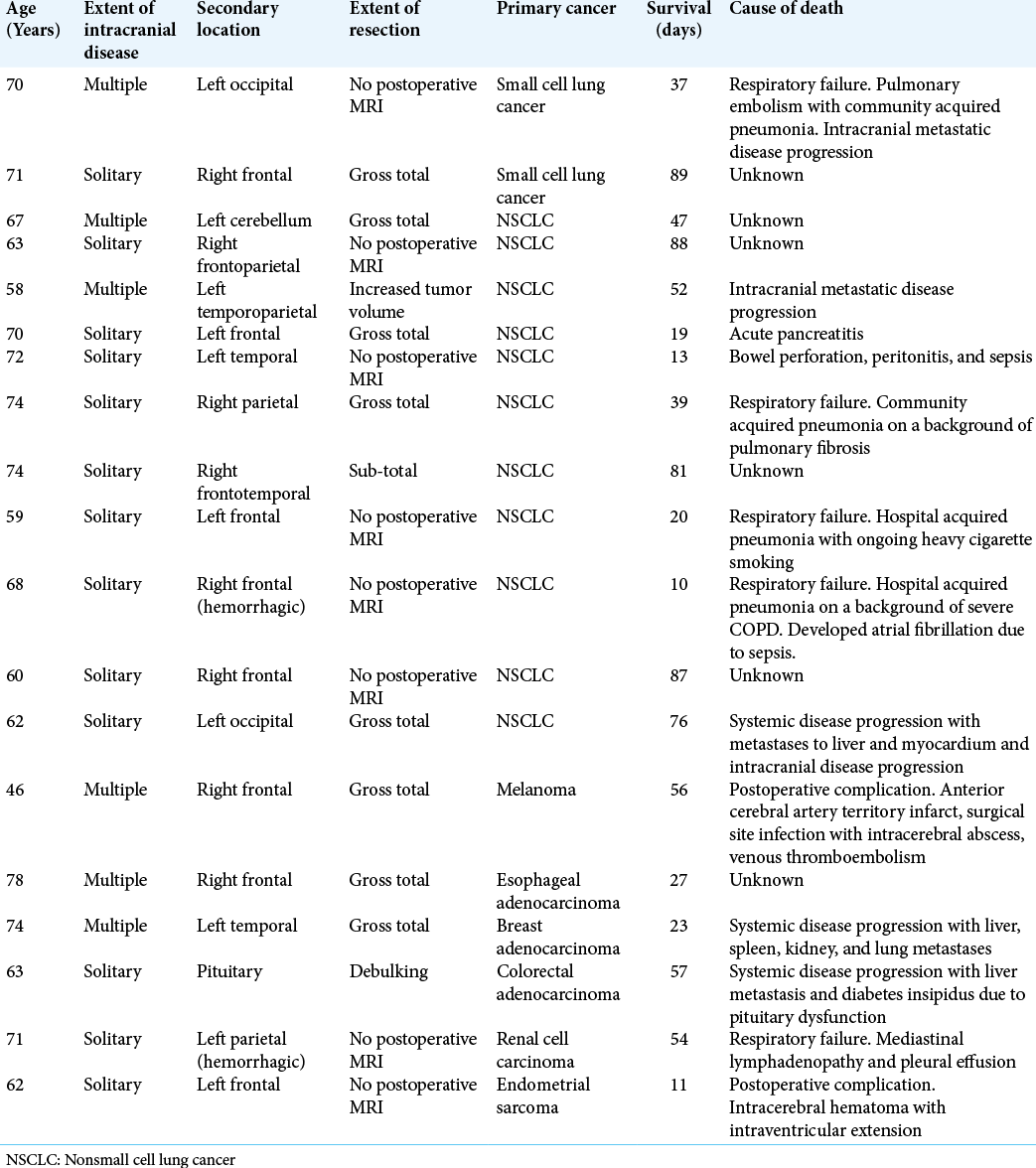
Of the 10 patients who underwent redo resection and/ or resection of an additional cranial metastatic lesion [
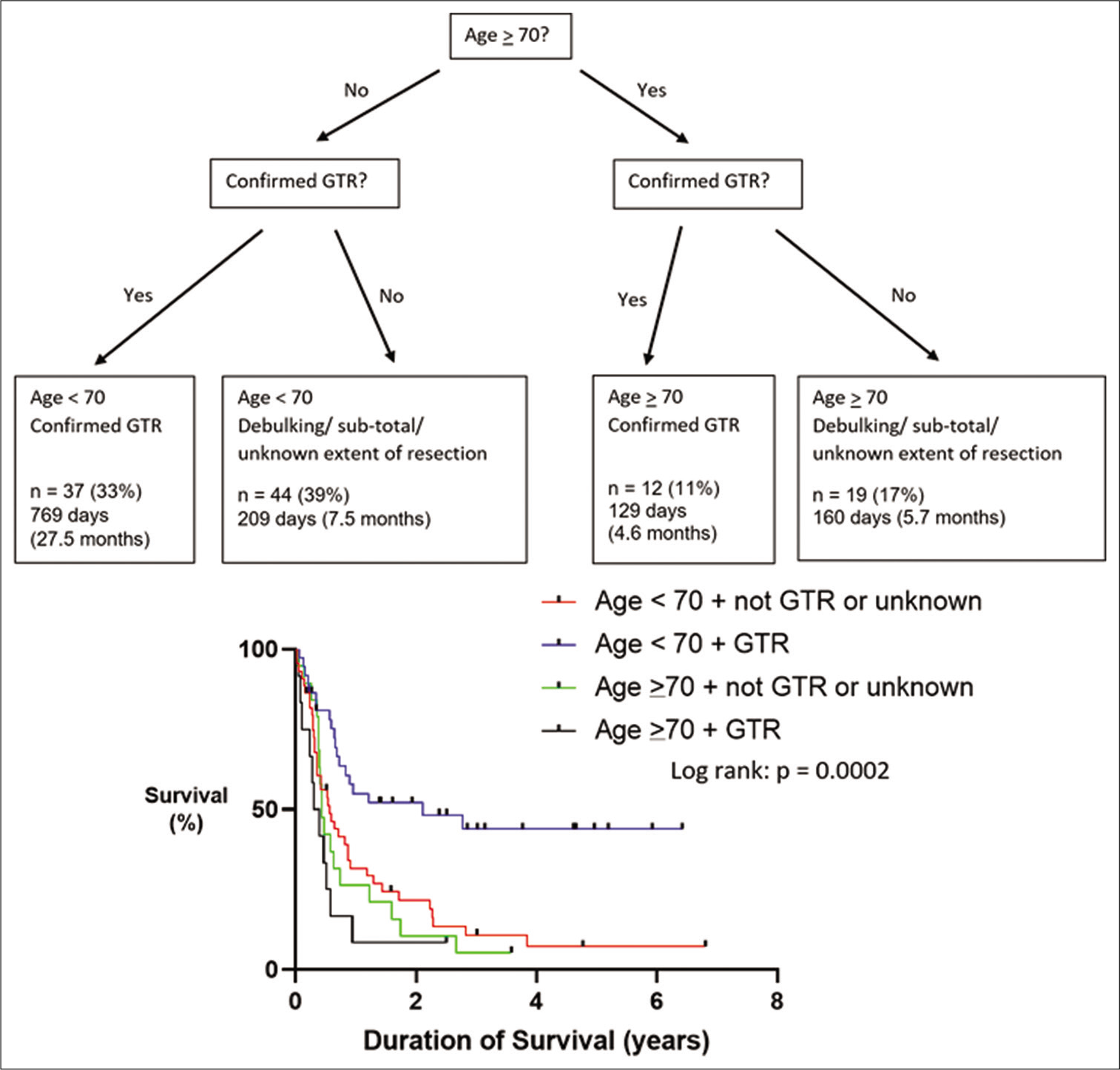
Age was significantly associated with survival (Log rank [Mantel–Cox] P = 0.009), with an age >70 being a negative prognostic predictor of survival [
DISCUSSION
Our study demonstrated that achieving gross-total resection can lead to prolonged survival. However, there was a large number of patients (37%) where MRI scanning was either delayed or not performed at all, resulting in patients with an unclear extent of surgical resection. Neurosurgical services in England are commissioned by the National Health Service (NHS) based on guidelines written by the National Institute for Health and Care Excellence (NICE).[
Schackert et al. found in their series of 127 patients; gross-total resection was associated with a longer duration of survival when compared to those with a residuum and median survivals of 10.6 and 5.8 months, respectively.[
Fluorescence-guided surgery using 5-aminolevulinic acid (5-ALA) has been shown to increase the likelihood of achieving gross-total resection in glioblastoma multiforme.[
Our study demonstrates that redo resection can be safely performed in patients with recurrent intracranial metastases with good overall survival. However, earlier detection of recurrent disease and treatment with STRS could obviate the need for redo resection. 2/10 patients who underwent redo resection had an unknown extent of resection following their primary surgery. On the other hand, 4/10 of those who underwent redo resection had an MRI scan demonstrating gross-total resection following the primary surgery [
Our study is limited by lacking postoperative Karnofsky performance status; this is likely a prognostic predictor of survival and can also affect a patient’s suitability for adjuvant radiation treatment. There was no patient reported quality of life assessment. Furthermore, we do not state the value in symptomatic relief provided by surgical resection, such as relieving headaches or improving neurological deficits secondary to mass effect and cerebral edema. We also do not present the details of systemic treatments such as chemotherapy, as this service is managed in a different hospital.
The dose and duration of dexamethasone administration are missing. Dexamethasone can provide symptomatic relief and reduce the risk of neurological deterioration while awaiting surgery. Hutchinson et al. showed in a randomized controlled clinical trial that dexamethasone was associated with a higher incidence of unfavorable outcome (moderately severe disability to dead) in patients with chronic subdural hematoma; in particular, dexamethasone was associated with a higher incidence of infection.[
Data on cigarette smoking were missing from our study. Concurrent cigarette smoking increases the risk of surgical site infection, pneumonia, and perioperative mortality.[
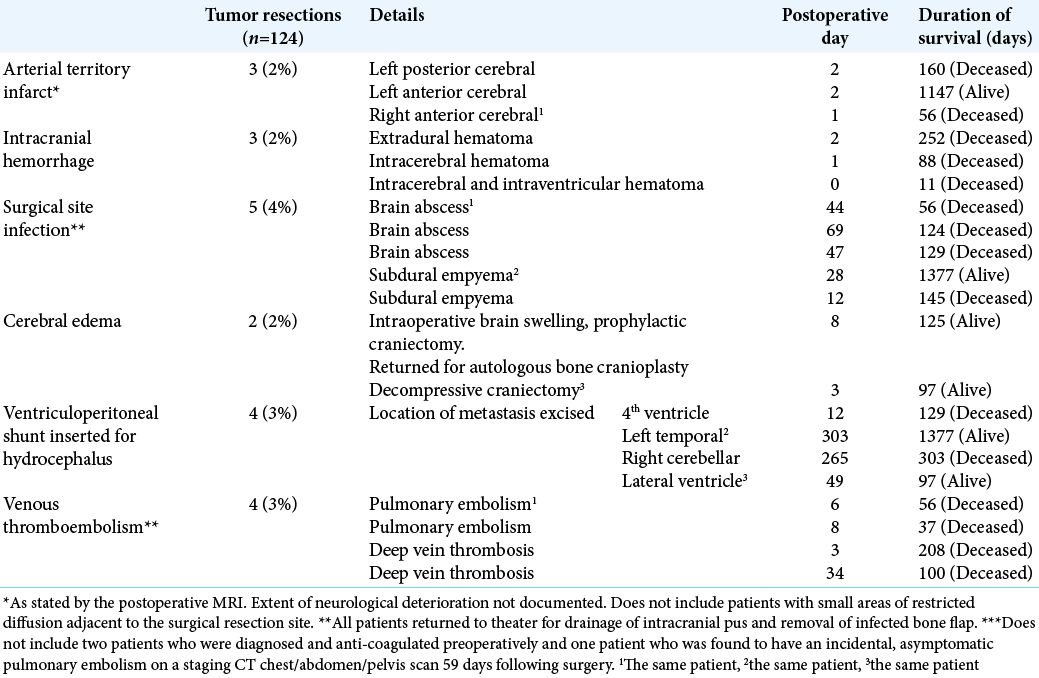
Death due to an unknown cause within 90 days of surgery occurred in 5% of the patients. These patients could have potentially died of a preventable cause such as surgical site infection, seizures, or venous thromboembolism. However, these patients may have chosen to not undergo further hospital admission, to focus on palliative symptomatic relief in their home. Furthermore, postmortem investigations are seldom performed in patients with metastatic cancer because it often does not add helpful information to the family members and can cause distress. While this is understandable, it is difficult to improve our service when there is missing information on the causes of postoperative death.
During the time period of data collection, there has been a change in the preferred adjuvant radiation treatment in our service. In 2017, Brown et al. demonstrated that when compared against WBRT, STRS is associated with a longer duration of cognitive-deterioration-free survival and no difference in overall survival for patients undergoing adjuvant radiation treatment to the surgical cavity following resection of a solitary brain metastasis.[
Given the impact of the COVID-19 pandemic on neurooncology services in the UK (37), this could have led to a reduction in overall survival for some patients in this study.
CONCLUSION
Cranial metastatic disease represents a heterogeneous patient population with multiple factors influencing the clinical outcome, therefore, when considering neurosurgical intervention, each case should be considered on an individual basis. There are ongoing advancements, with new medical therapies becoming available for different types of cancer in which neurosurgeons may not be aware of. Therefore, input from oncologists who treat the primary disease is crucial when selecting patients who would be suitable candidates for neurosurgical intervention. Early postoperative MRI scanning is recommended to identify residual tumor or new discrete lesions which could benefit from adjuvant STRS. If required, redo resection can successfully be performed with benefits to overall survival. In our study, age and extent of surgical resection were prognostic predictors of survival.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY FIGURE
References
1. Al-Shamy G, Sawaya R. Management of brain metastases: The indispensable role of surgery. J Neurooncol. 2009. 92: 275-82
2. Banfill KE, Bownes PJ, St Clair SE, Loughrey C, Hatfield P. Stereotactic radiosurgery for the treatment of brain metastases: Impact of cerebral disease burden on survival. Br J Neurosurg. 2012. 26: 674-8
3. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017. 18: 1049-60
4. D’Andrea G, Palombi L, Minniti G, Pesce A, Marchetti P. Brain metastases: Surgical treatment and overall survival. World Neurosurg. 2017. 97: 169-77
5. Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012. 14: 1171-7
6. de Lima Oliveira M, Picarelli H, Menezes MR, Amorim RL, Teixeira MJ, Bor-Seng-Shu E. Ultrasonography during surgery to approach cerebral metastases: Effect on karnofsky index scores and tumor volume. World Neurosurg. 2017. 103: 557-65
7. Du YJ, Pahernik S, Hadaschik B, Teber D, Duensing S, Jäger D. Impact of resection and systemic therapy on the survival of patients with brain metastasis of metastatic renal cell carcinoma. J Neurooncol. 2016. 130: 221-8
8. Fox BD, Cheung VJ, Patel AJ, Suki D, Rao G. Epidemiology of metastatic brain tumors. Neurosurg Clin N Am. 2011. 22: 1-6
9. Gavrilovic IT, Posner JB. Brain metastases: Epidemiology and pathophysiology. J Neurooncol. 2005. 75: 5-14
10. Gupta S, Dawood H, Larsen AG, Fandino L, Knelson EH, Smith TR. Surgical and peri-operative considerations for brain metastases. Front Oncol. 2021. 11: 662943
11. Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol. 2000. 17: 279-86
12. Hussein A, Rohde V, Wolfert C, Hernandez-Duran S, Fiss I, Bleckmann A. Survival after resection of brain metastases with white light microscopy versus fluorescence-guidance: A matched cohort analysis of the Metastasys study data. Oncotarget. 2020. 11: 3026-34
13. Hutchinson PJ, Edlmann E, Bulters D, Zolnourian A, Holton P, Suttner N. Trial of dexamethasone for chronic subdural hematoma. N Engl J Med. 2020. 383: 2616-27
14. Jung M, Ahn JB, Chang JH, Suh CO, Hong S, Roh JK. Brain metastases from colorectal carcinoma: Prognostic factors and outcome. J Neurooncol. 2011. 101: 49-55
15. Jünger ST, Pennig L, Schödel P, Goldbrunner R, Friker L, Kocher M. The debatable benefit of gross-total resection of brain metastases in a comprehensive treatment setting. Cancers (Basel). 2021. 13: 1435
16. Kamp MA, Fischer I, Bühner J, Turowski B, Cornelius JF, Steiger HJ. 5-ALA fluorescence of cerebral metastases and its impact for the local-in-brain progression. Oncotarget. 2016. 7: 66776-89
17. Kamp MA, Munoz-Bendix C, Mijderwijk HJ, Turowski B, Dibué-Adjei M, von Saß C. Is 5-ALA fluorescence of cerebral metastases a prognostic factor for local recurrence and overall survival?. J Neurooncol. 2019. 141: 547-53
18. Lau D, Ziewacz JE, Siddiqi HK, Pelly A, Sullivan SE, El-Sayed AM. Cigarette smoking: A risk factor for postoperative morbidity and 1-year mortality following craniotomy for tumor resection. J Neurosurg. 2012. 116: 1204-14
19. Massard C, Zonierek J, Gross-Goupil M, Fizazi K, Szczylik C, Escudier B. Incidence of brain metastases in renal cell carcinoma treated with sorafenib. Ann Oncol. 2010. 21: 1027-31
20. McCutcheon IE. Colorectal carcinoma and brain metastasis: Distribution, treatment, and survival. Ann Surg Oncol. 1996. 3: 453-63
21. Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011. 385: 650-1
22. NHS England.editors. Clinical Commissioning Policy: Stereotactic Radiosurgery (SRS) and Stereotactic Radiotherapy (SRT) to the Surgical Cavity Following Resection of Cerebral Metastases (All ages) [P200902P] (URN: 1857). 2021. p.
23. 24. Nolan MB, Martin DP, Thompson R, Schroeder DR, Hanson AC, Warner DO. Association between smoking status, preoperative exhaled carbon monoxide levels, and postoperative surgical site infection in patients undergoing elective surgery. JAMA Surg. 2017. 152: 476-83 25. Olesrud IC, Schulz MK, Marcovic L, Kristensen BW, Pedersen CB, Kristiansen C. Early postoperative MRI after resection of brain metastases complete tumour resection associated with prolonged survival. Acta Neurochir (Wien). 2019. 161: 555-65 26. Paek SH, Audu PB, Sperling MR, Cho J, Andrews DW. Reevaluation of surgery for the treatment of brain metastases: Review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery. 2005. 56: 1021-34 27. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. J Am Med Assoc. 1998. 280: 1485-9 28. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322: 494-500 29. Pessina F, Navarria P, Cozzi L, Ascolese AM, Maggi G, Rossi M. Role of surgical resection in patients with single large brain metastases: Feasibility, morbidity, and local control evaluation. World Neurosurg. 2016. 94: 6-12 30. Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006. 17: 935-44 31. Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015. 372: 30-9 32. Samlowski WE, Moon J, Witter M, Atkins MB, Kirkwood JM, Othus M. High frequency of brain metastases after adjuvant therapy for high-risk melanoma. Cancer Med. 2017. 6: 2576-85 33. Sampson JH, Carter JH, Friedman AH, Seigler BF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998. 88: 11-20 34. Schackert G, Lindner C, Petschke S, Leimert M, Kirsch M. Retrospective study of 127 surgically treated patients with multiple brain metastases: Indication, prognostic factors, and outcome. Acta Neurochir (Wien). 2013. 155: 379-87 35. Schödel P, Jünger ST, Wittersheim M, Reinhardt HC, Schmidt NO, Goldbrunner R. Surgical resection of symptomatic brain metastases improves the clinical status and facilitates further treatment. Cancer Med. 2020. 9: 7503-10 36. Schödel P, Schebesch KM, Brawanski A, Proescholdt MA. Surgical resection of brain metastases-impact on neurological outcome. Int J Mol Sci. 2013. 14: 8708-18 37. Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002. 94: 2698-705 38. Sivasanker M, Madhugiri VS, Moiyadi AV, Shetty P, Subi TS. Surgery for brain metastases: An analysis of outcomes and factors affecting survival. Clin Neurol Neurosurg. 2018. 168: 153-62 39. Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A. Estimating survival in patients with lung cancer and brain metastases an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol. 2017. 3: 827-31 40. Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme utilizing 5-ALA-induced porphyrins: A prospective study in 52 consecutive patients. J Neurosurg. 2000. 93: 1003-13 41. Suarez-Sarmiento A, Nguyen KA, Syed JS, Nolte A, Ghabili K, Cheng M. Brain metastasis from renal-cell carcinoma: An institutional study. Clin Genitourin Cancer. 2019. 17: e1163-70 42. Tendulkar RD, Liu SW, Barnett GH, Vogelbaum MA, Toms SA, Jin T. RPA classification has prognostic significance for surgically resected single brain metastasis. Int J Radiat Oncol Biol Phys. 2006. 66: 810-7 43. Vosoughi E, Lee JM, Miller JR, Nosrati M, Minor DR, Abendroth R. Survival and clinical outcomes of patients with melanoma brain metastasis in the era of checkpoint inhibitors and targeted therapies. BMC Cancer. 2018. 18: 490 44. Yagi R, Kawabata S, Ikeda N, Nonoguchi N, Furuse M, Katayama Y. Intraoperative 5-aminolevulinic acid-induced photodynamic diagnosis of metastatic brain tumors with histopathological analysis. World J Surg Oncol. 2017. 15: 179 45. Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS. Reduced local recurrence of a single brain metastasis through microscopic total resection: Clinical article. J Neurosurg. 2009. 110: 730-6