- Department of Neurosurgery, Geisinger Health System, Danville, PA, USA
- Department of Neurosurgery, Geisinger Health System, Wilkes-Barre, PA, USA.
Correspondence Address:
Oded Goren, Department of Neurosurgery, Geisinger Health System, Danville, PA, USA.
DOI:10.25259/SNI_1151_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Philipp Hendrix1,2, Christian Bohan1, Shamsher Singh Dalal1, Gregory M. Weiner2, Ulrick S. Kanmounye1, Clemens M. Schirmer1,2, Oded Goren1. Proper ophthalmic artery aneurysms. 24-Mar-2023;14:105
How to cite this URL: Philipp Hendrix1,2, Christian Bohan1, Shamsher Singh Dalal1, Gregory M. Weiner2, Ulrick S. Kanmounye1, Clemens M. Schirmer1,2, Oded Goren1. Proper ophthalmic artery aneurysms. 24-Mar-2023;14:105. Available from: https://surgicalneurologyint.com/surgicalint-articles/12208/
Abstract
Background: The ophthalmic segment of the internal carotid artery (ICA) represents a common site for cerebral aneurysms. However, aneurysms of the ophthalmic artery (OphA) itself represent rare lesions and have been associated with trauma and flow-related lesions such as arteriovenous fistulas or malformations. Here, we explore clinical and radiological features of four patients managed for five proper ophthalmic artery aneurysms (POAAs).
Methods: Patients undergoing diagnostic cerebral angiogram (DCA) between January 2018 and November 2021 with newly or previously identified POAA were retrospectively reviewed. Clinical and radiological data were analyzed to identify common and unique features.
Results: Four patients with identification of five POAA were identified. Three patients suffered traumatic brain injury with subsequent identification of POAA on DCA. Patient 1 presented with a traumatic carotid-cavernous-sinus fistula requiring transvenous coil embolization and second stage flow diversion of the ICA. Patient 2 suffered a gunshot wound with ICA compromise, ethmoidal dural arteriovenous fistula (dAVF) development with rapid growth of two POAAs eventually requiring Onyx embolization. Patient 3 was assaulted and DCA showed a POAA without any other cerebrovascular pathology. Patient 4 had undergone N-butyl cyanoacrylate embolization of an ethmoidal dAVF 13 years ago with the feeding OphA carrying a large POAA. Re-DCADCA was performed for a newly developed and unrelated transverse-sigmoid-sinus dAVF.
Conclusion: Management of POAAs poses a challenge to neurovascular surgeons since POAAs inherit a risk for visual deterioration or hemorrhage. DCA facilitates identification of coexisting cerebrovascular pathology. If clinically silent and not accompanied by cerebrovascular disease, observation appears reasonable.
Keywords: Aneurysm, Dural arteriovenous fistula, Ophthalmic artery
INTRODUCTION
The term ophthalmic artery (OphA) aneurysm is commonly used to delineate ophthalmic segment internal carotid artery (ICA) aneurysms. Terminology for aneurysms of the OphA itself is diverse. Terms such as proper, true, itself, and stem have been used. However, those terms often do not appropriately display anatomical or histological features. Qiao et al. utilized the term peripheral OphA aneurysm. In their report, intracranial, intracanalicular, intraorbital, and terminal aneurysms of the proper OphA have been reviewed.[
MATERIALS AND METHODS
Exploring the institutional prospectively maintained case log, a total of four cases with newly or previously identified POAAs between January 2018 and June 2021 were found. In the study period, 3542 diagnostic cerebral angiograms (DCAs) in 2704 patients were performed. Local institutional review board (IRB) approval was obtained for retrospective analysis (IRB #2021-0901), patient consent was waived. The herein presented study is in accordance with ethical standards of the Declaration of Helsinki. Clinical and radiological data from the electronic medical records were reviewed.
RESULTS
Patient 1
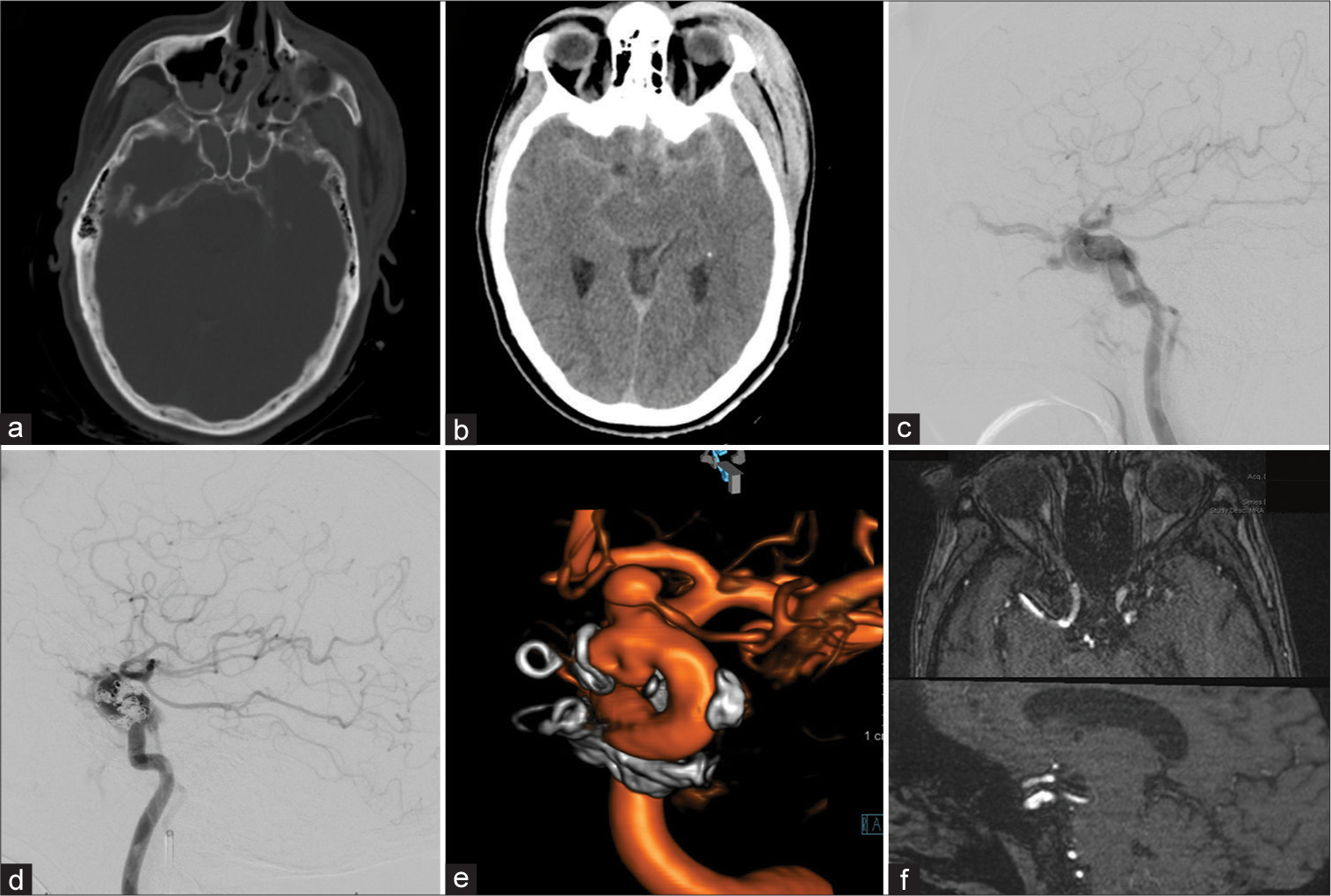
A 55-year-old female presented after a motor bicycle accident with subarachnoid hemorrhage, bilateral subdural hematoma pronounced around the tentorial leaflets, bilateral sphenoid, nasal septal, and left zygomaticomaxillary complex fractures with an associated pansinus hemorrhage and a large left scalp hematoma [
Figure 1:
Patient 1, 55 F, traumatic brain injury. Admission computed tomography-head (a and b). Type A carotid-cavernous fistula in lateral diagnostic cerebral angiogram (DCA)-projection, the retrograde filling of the left superior ophthalmic vein is evident (c). Transvenous coil-embolization was performed in the acute setting (d). Follow-up DCA after 8 months showed a dorsal-medial focal outpouching of the internal carotid artery (ICA) as well as a prominent intracranial proper ophthalmic artery aneurysm (e). Flow diversion using Pipeline embolization device was performed and 6-month follow-up magnetic resonance angiography shows resolution of the ICA defect (f).
Patient 2
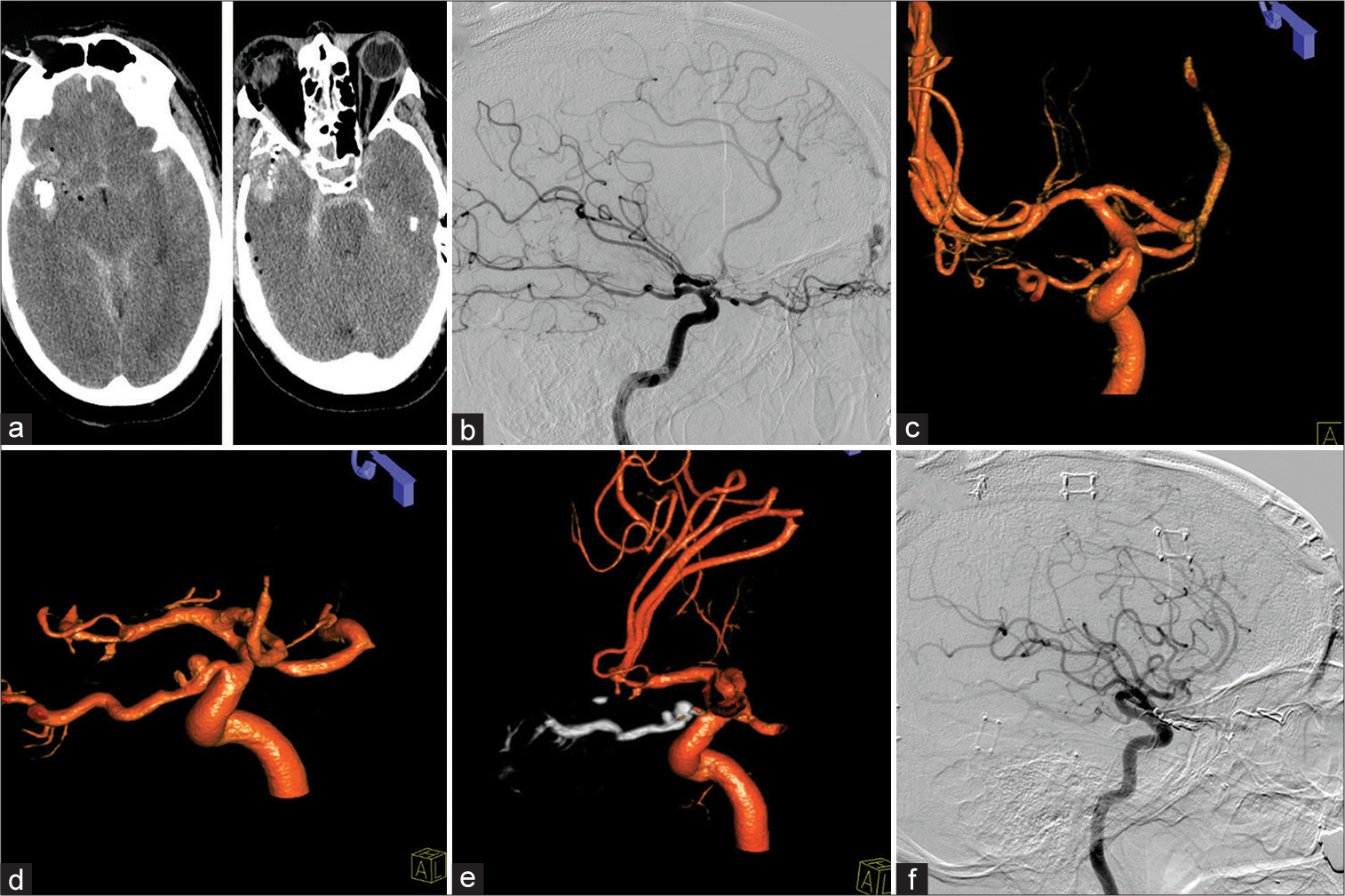
A 58-year-old male suffered a right-sided temporal gunshot wound. The projectile tract had a downward trajectory extending through the right posterior orbit, nasal septum, and left maxillary sinus with sparing of the left orbit [
Figure 2:
Patient 2, 58 M, right temporal gunshot wound. Admission computed tomography-head (a). A traumatic right-sided ethmoidal dural arteriovenous fistula (dAVF) was evident on lateral diagnostic cerebral angiogram (b). Two minimal outpouchings of the intracranial and intracanalicular segment of the ophthalmic artery were identified as well (c). Three weeks after a complicated course, both proper ophthalmic artery aneurysm (POAAs) grew (d). Treatment of dAVF as well as POAAs was performed through Onyx embolization (e) with successful resolution of the POAAs as well as dAVF (f).
Patient 3
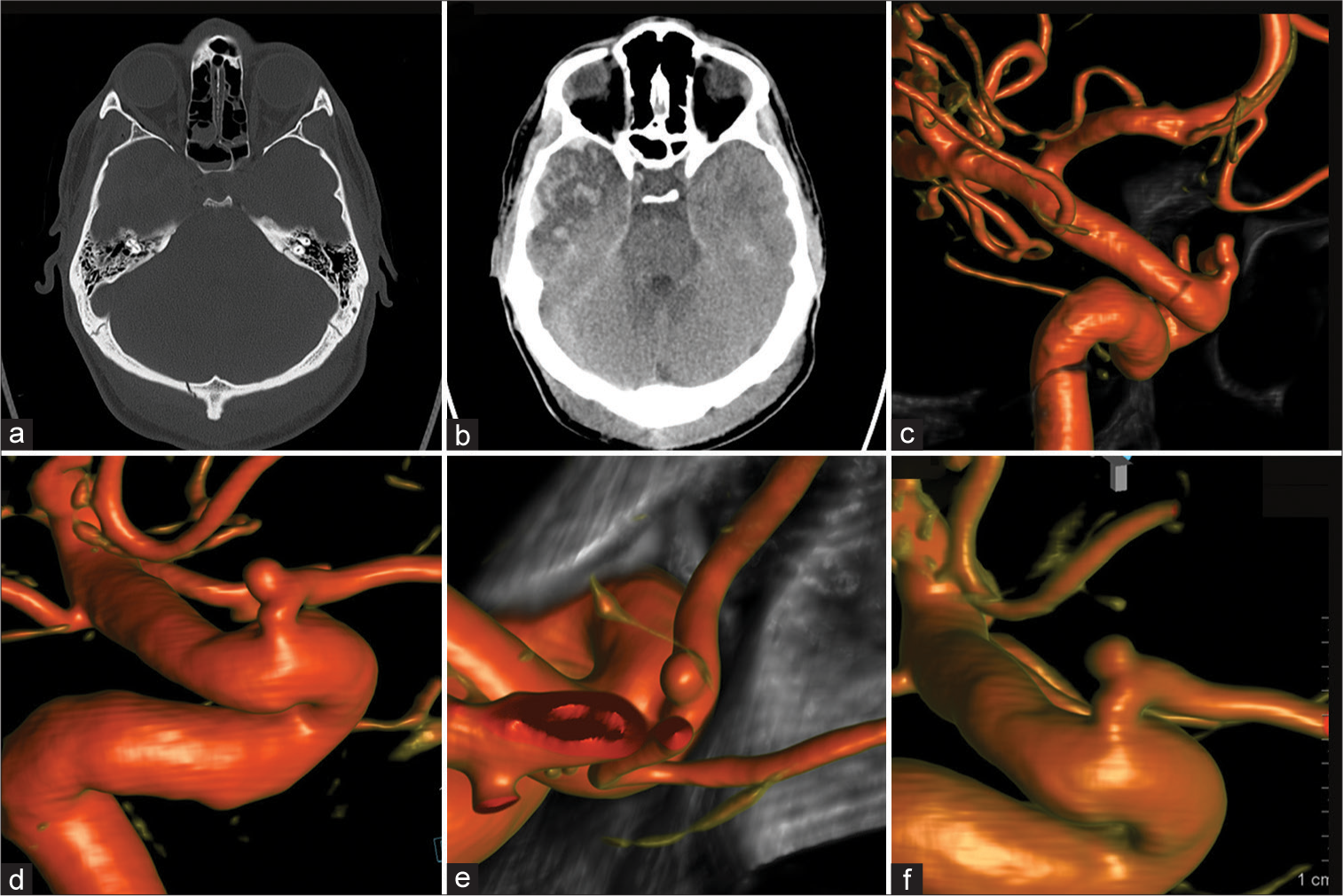
A 31-year-old male was assaulted with a metallic objected and suffered a strike against the head. He was admitted to an outside hospital and a non contrast head CT imaging showed an occipital skull fracture, small falcine SDH, and a right temporal contusion [
Figure 3:
Patient 3, 31 M, traumatic brain injury. Admission computed tomography-head (a and b). Four-month follow-up diagnostic cerebral angiogram (DCA) for potential vascular compromise was performed revealing a small intracranial proper ophthalmic artery aneurysm (c). Subsequent 4, 9, and 21 months DCA follow-ups demonstrated stable aneurysm size and morphology (d-f, respectively).
Patient 4
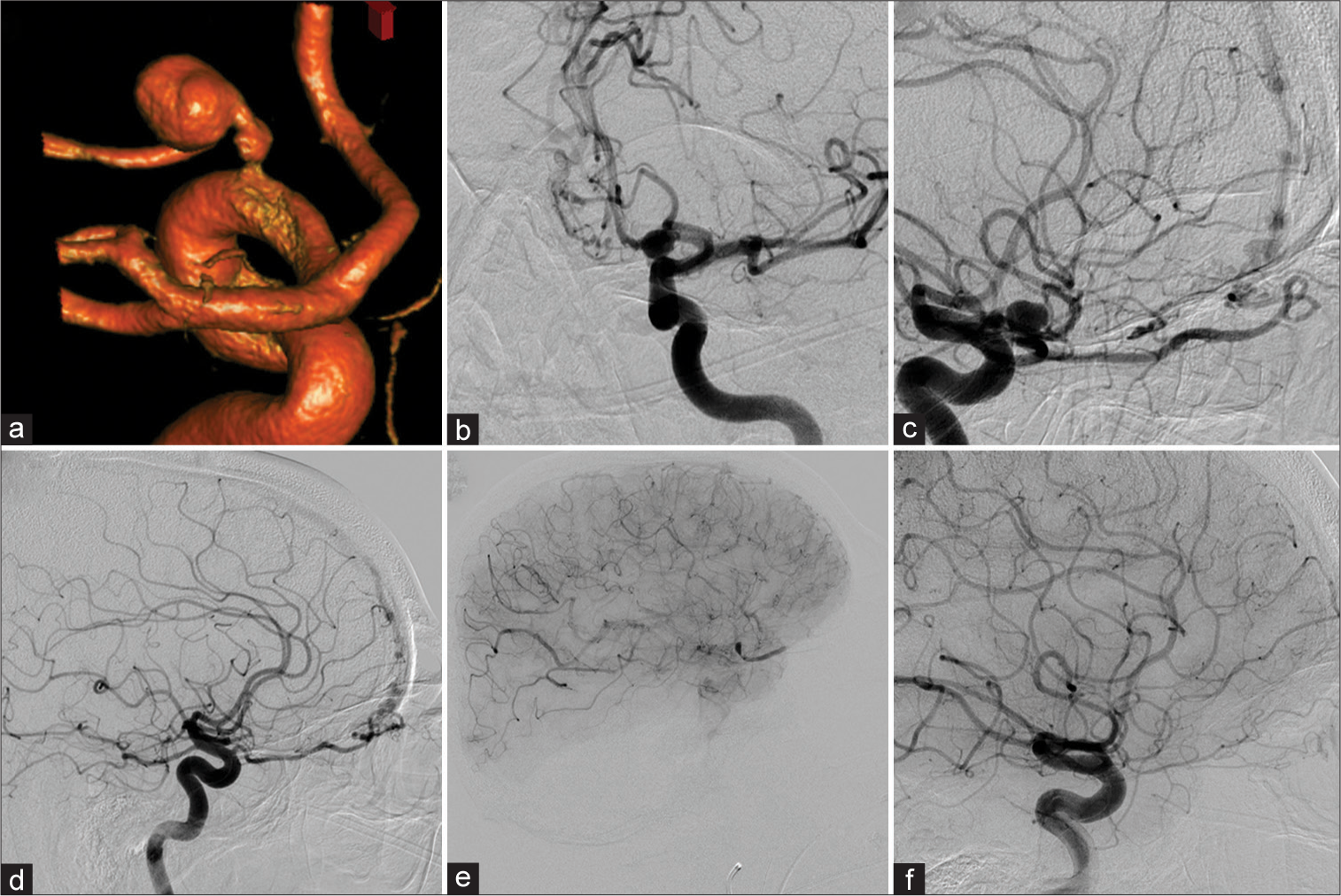
In 2008, a 68-year-old male patient complaining of retro-orbital pain was found to have a left POAA on a CT scan of the orbits. A magnetic resonance imaging and MRA were completed that redemonstrated the aneurysm. The subsequent DCA showed the aneurysm arising from the left OphA [
Figure 4:
Patient 4 was diagnosed with a left-sided proper ophthalmic artery aneurysm (POAA) (a-c) in a setting of a left (b and c) and right (d) sided ethomoidal dural arterio-venous fistula. After right-sided N-butyl cyanoacrylate (NBCA) embolization, the left side was treated with NBCA as well. However, the ophthalmic artery (OphA) demonstrated contrast stasis in the intraorbital segment – as well as in the POAA – in the postembolization run (e). Follow-up diagnostic cerebral angiogram (DCA) 5 months later revealed reconstitution of a small calibered OphA. However, vision did not recover beyond light-dark perception. Twelve years later, the DCA demonstrated equal findings (f).
DISCUSSION
To date, several case reports of POAAs have been published. Still, POAAs remain poorly understood and thus pose a challenge if identified on cerebrovascular imaging. POAAs have been identified arising from all OphA segments with the intracanalicular location representing the least common site, while the intracranial and the intraorbital locations appear to be the most prominent.[
Limitation
The small series of POAAs bases on imaging reports during the study period. However, the herein identified patient count with POAAs per patient-angiograms in the study period cannot reliably be used to estimate a prevalence of POAAs. Not every patient underwent 3D-rotational angiography and small POAAs might easily be overlooked without proper angiographic assessment of the OphA.
CONCLUSION
POAAs represent a rare entity that may go unnoticed in patients suffering from traumatic brain injury or cerebrovascular pathologies. Still, they do pose a risk for visual deterioration or even hemorrhage. This report adds to the scant literature on POAAs and may help informing clinical decision-making.
Ethical statement
Ethical approval: Approval was obtained by the local IRB #2021-0901 Informed consent: waived by IRB for retrospective analysis.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Cagnazzo F, Peluso A, Vannozzi R, Brinjikji W, Lanzino G, Perrini P. Arterial aneurysms associated with intracranial dural arteriovenous fistulas: Epidemiology, natural history, and management. A systematic review. Neurosurg Rev. 2019. 42: 277-85
2. Choi BK, Lee TH, Choi CH, Lee SW. Fusiform intracanalicular ophthalmic artery aneurysm; Case report and review of literature. J Korean Neurosurg Soc. 2008. 44: 43-6
3. Chun HJ, Yi HJ. Traumatic extracranial pseudoaneurysm on the peripheral ophthalmic artery presenting as delayed intraparenchymal hematoma: Case report. Surg Neurol. 2009. 71: 701-4
4. Dehdashti AR, Safran AB, Martin JB, Rüfenacht DA, de Tribolet N. Intraorbital ophthalmic artery aneurysm associated with basilar tip saccular aneurysm. Neuroradiology. 2002. 44: 600-3
5. Foreman PM, Hendrix P, Harrigan MR, Fisher WS, Vyas NA, Lipsky RH. PHASES score applied to a prospective cohort of aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. 2018. 53: 69-73
6. Griessenauer CJ, Farrell S, Sarkar A, Zand R, Abedi V, Holland N. Genetic susceptibility to cerebrovascular disease: A systematic review. J Cereb Blood Flow Metab. 2018. 38: 1853-71
7. Gross BA, Ropper AE, Du R. Cerebral dural arteriovenous fistulas and aneurysms. Neurosurg Focus. 2012. 32: E2
8. Hendrix P, Griessenauer CJ, Foreman P, Loukas M, Fisher WS, Rizk E. Arterial supply of the lower cranial nerves: A comprehensive review. Clin Anat. 2014. 27: 108-17
9. Hendrix P, Griessenauer CJ, Foreman P, Shoja MM, Loukas M, Tubbs RS. Arterial supply of the upper cranial nerves: A comprehensive review. Clin Anat. 2014. 27: 1159-66
10. Hendrix P, Griessenauer CJ, Foreman P, Shoja MM, Tubbs RS, editors. Blood supply of the cranial nerves. Nerves and Nerve Injuries. Netherlands: Elsevier Science; 2015. p. 427-38
11. Kawaguchi S, Sakaki T, Okuno S, Uchiyama Y, Nishioka T. Peripheral ophthalmic artery aneurysm. Report of two cases. J Neurosurg. 2001. 94: 822-5
12. Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations in the circle of Willis. Stroke. 1984. 15: 846-50
13. Martínez-Pérez R, Tsimpas A, Ruiz Á, Montivero A, Mura J. Spontaneous regression of a true intracanalicular fusiform ophthalmic artery aneurysm after endovascular treatment of an associated dural arteriovenous fistula. World Neurosurg. 2018. 119: 362-5
14. Meisel HJ, Mansmann U, Alvarez H, Rodesch G, Brock M, Lasjaunias P. Cerebral arteriovenous malformations and associated aneurysms: Analysis of 305 cases from a series of 662 patients. Neurosurgery. 2000. 46: 793-800 discussion 800-2
15. Pandey P, Rayes M, Guthikonda M, Xavier A. Peripheral ophthalmic artery aneurysm associated with multiple intracranial aneurysms: A case report. J Neurointerv Surg. 2010. 2: 211-2
16. Peschillo S, Biraschi F, Diana F, Colonnese C, Marenco M, Delfini R. Aneurysms of the intracranial segment of the ophthalmic artery trunk: Case report and systematic literature review. J Neurol Surg A Cent Eur Neurosurg. 2018. 79: 257-61
17. Piché SL, Haw CS, Redekop GJ, Heran MK. Rare intracanalicular ophthalmic aneurysm: Endovascular treatment and review of the literature. AJNR Am J Neuroradiol. 2005. 26: 1929-31
18. Qiao L, Wang H, Mao L, Chen S, Xie W, Wu Q. Peripheral ophthalmic artery aneurysm. Neurosurg Rev. 2011. 34: 29-38
19. Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: Classification, incidence, and risk of hemorrhage. J Neurosurg. 1998. 89: 539-46
20. Sato S, Suga S, Ohira T, Takayama H, Kawase T. Aneurysm of the ophthalmic artery trunk. Acta Neurochir (Wien). 1999. 141: 321-2
21. Sirakov S, Sirakov A, Tsonev H, Hristov H. Ruptured intracanalicular ophthalmic artery aneurysm treated with low profile flow diverter device: Case report. Clin Neuroradiol. 2020. 30: 177-80
22. Yanaka K, Matsumaru Y, Kamezaki T, Nose T. Ruptured aneurysm of the ophthalmic artery trunk demonstrated by three-dimensional rotational angiography: Case report. Neurosurgery. 2002. 51: 1066-9 discussion 1069-70
23. Zheng Y, Lu Z, Shen J, Xu F. Intracranial pseudoaneurysms: Evaluation and management. Front Neurol. 2020. 11: 582