- School of Medicine, Baylor College of Medicine, Houston, Texas, United States.
- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
- Department of Pathology and Immunology, Baylor College of Medicine, Houston, Texas, United States.
- Department of Neurology, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Akash J. Patel, Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
DOI:10.25259/SNI_852_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Adin M. Ehrlich1, Michael Benjamin Larkin2, Collin William English2, Arya Shetty2, Mayuri Gupta2, Shervin Hosseingholi Nouri2, Hsiang-Chih Lu3, Jacob J. Mandel4, Akash J. Patel2. Protracted course of chemical meningitis following posterior fossa epidermoid cyst excision – A case report. 18-Nov-2022;13:544
How to cite this URL: Adin M. Ehrlich1, Michael Benjamin Larkin2, Collin William English2, Arya Shetty2, Mayuri Gupta2, Shervin Hosseingholi Nouri2, Hsiang-Chih Lu3, Jacob J. Mandel4, Akash J. Patel2. Protracted course of chemical meningitis following posterior fossa epidermoid cyst excision – A case report. 18-Nov-2022;13:544. Available from: https://surgicalneurologyint.com/surgicalint-articles/12016/
Abstract
Background: Chemical meningitis, a subtype of aseptic meningitis, as a complication of posterior fossa surgery is not a rare complication. However, the description of a severe protracted course following the surgical resection of an epidermoid cyst has not been described in the current literature. Chemical meningitis is thought to be associated with a hyperreactive inflammatory response, mediated in part by interleukin (IL)-10, IL-1β, and tumor necrosis factor-α, to the postoperative keratin debris from the spontaneous leakage or surgical release of epidermoid contents into subarachnoid spaces, which ultimately can result in patient symptoms of meningitis and hydrocephalus. Often, this remains mild and the recommended management includes a short course administration of corticosteroids.
Case Description: The authors report such a case in a patient who underwent a redoresection for a fourth ventricular epidermoid cyst. Postoperatively, the patient returned several times with symptoms of meningitis and hydrocephalus requiring multiple hospitalizations in the ensuing months. The patient required emergent cerebrospinal fluid diversion, further posterior fossa exploration and an extended high-dose corticosteroid treatment regimen.
Conclusion: The authors summarize the current understanding of the biochemical processes involved for the rare presentation of postoperative chemical meningitis.
Keywords: Aseptic meningitis, Chemical meningitis, Epidermoid cyst, Neurosurgery, Posterior fossa
INTRODUCTION
Epidermoid cysts are benign lesions that occur due to a disorder in neural tube closure during development, in which epithelial remnants are trapped inside neural tissue.[
Illustrative case
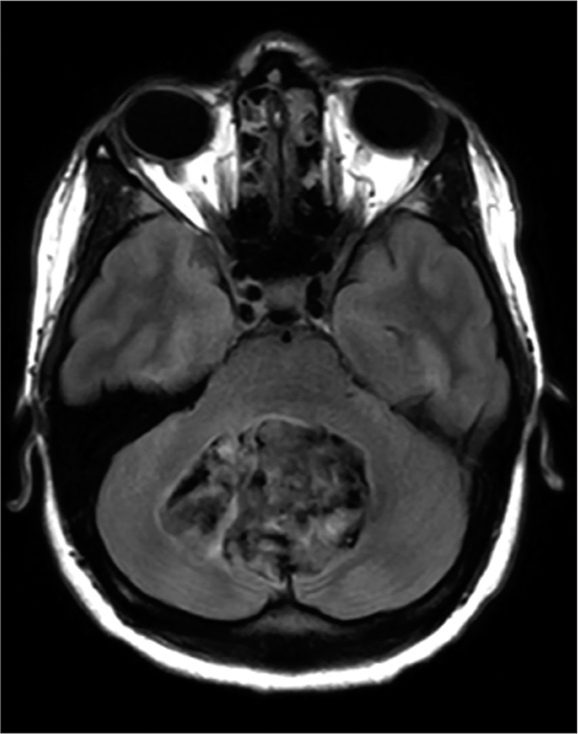
A 33-year-old woman underwent a redoresection for a fourth ventricular epidermoid cyst which had previously been operated on 4 years prior [
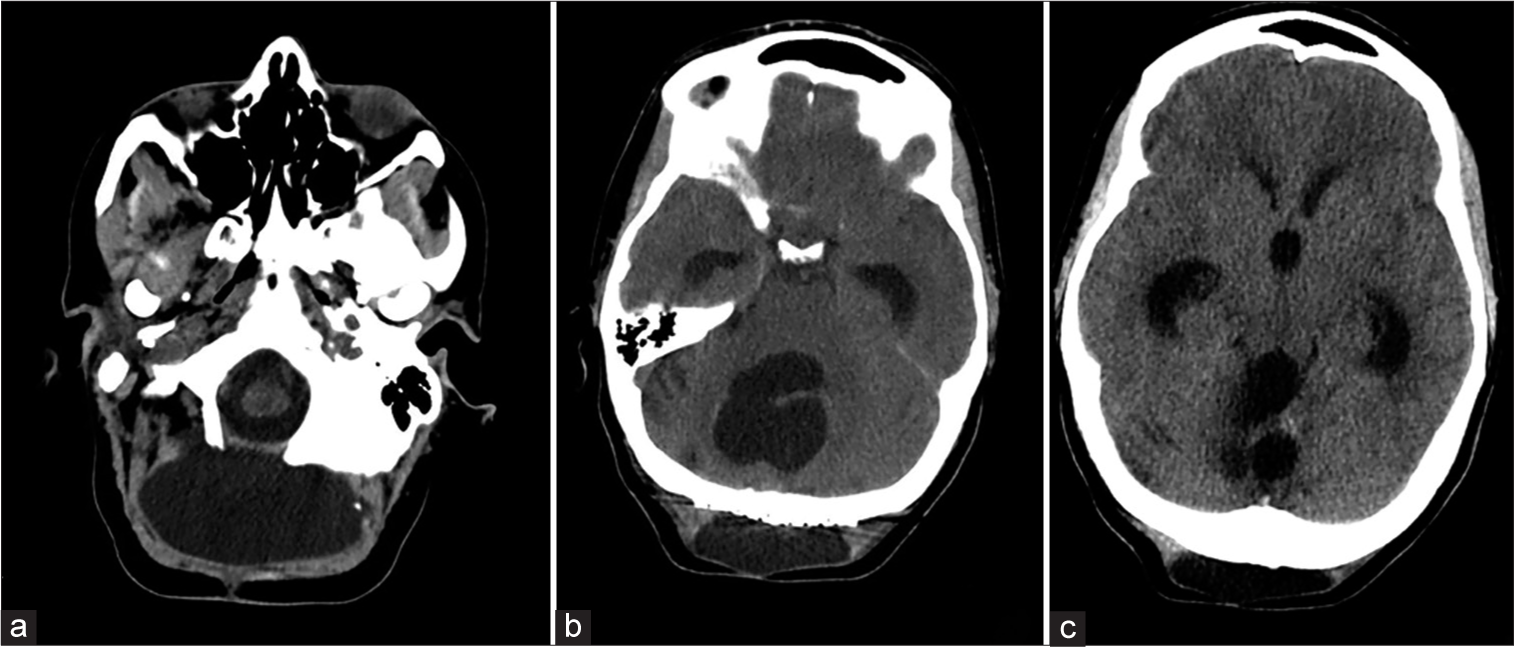
One month later, the patient returned with nausea, vomiting, and intermittent fevers of up to 101°F. Computed tomography (CT) of the brain demonstrated the formation of a suboccipital pseudomeningocele. There was no wound dehiscence and following an additional short course steroid taper administration that the patient’s headache and nausea improved.
Two weeks later, she once again presented with worsening headache, nausea, but this time with episodes of confusion, fever, and chills. A repeat CT showed development of moderate hydrocephalus and the progressive enlargement of the suboccipital pseudomeningocele [
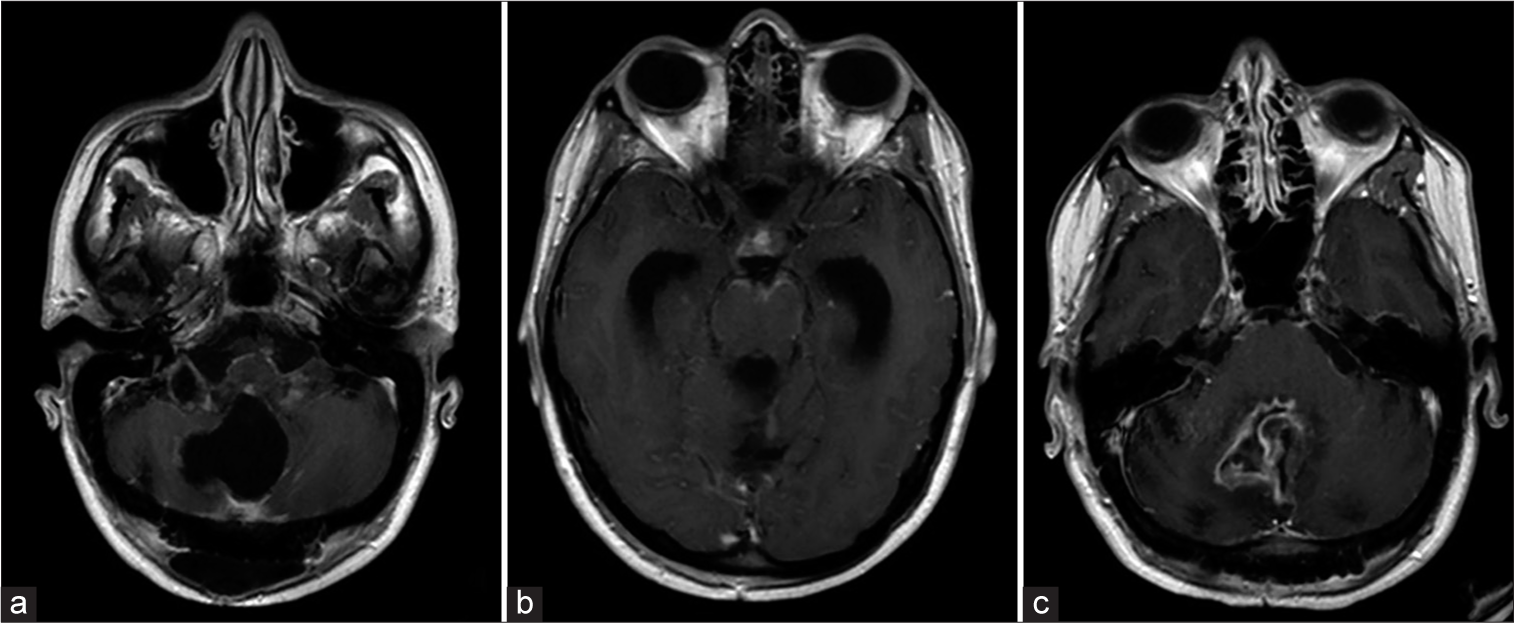
Afterward, the patient remained in the hospital and continued to exhibit intermittent fevers. Several days postoperatively, she developed an acute onset right facial droop and double vision. A repeat MRI showed further progression of the leptomeningeal enhancement concerning for worsening meningitis; however, repeat CSF Gram stain and culture remained negative. Over the coming days, the patient’s symptoms improved and she was again started on a course of steroids for chemical meningitis and discharged home following a 3-week admission.
Two days later, the patient returned to the emergency department with acute onset symptoms of VPS failure and underwent an emergent revision of the VPS. She quickly improved following shunt revision and was again discharged home when appropriate.
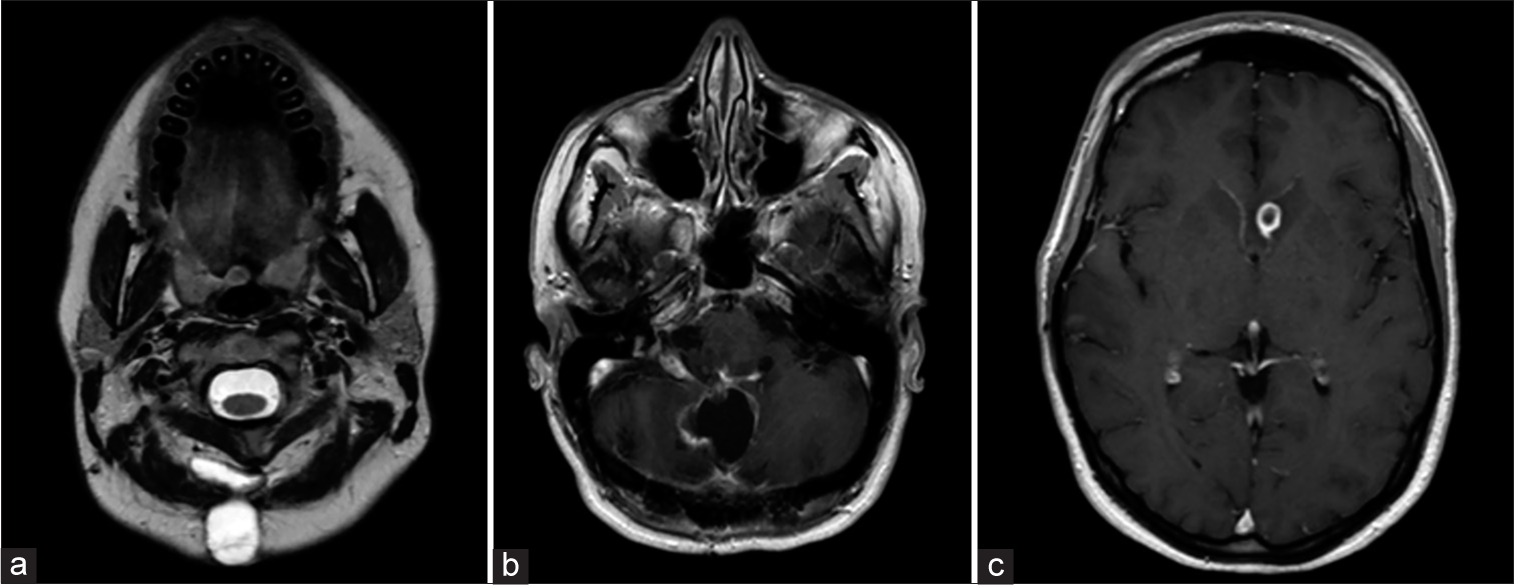
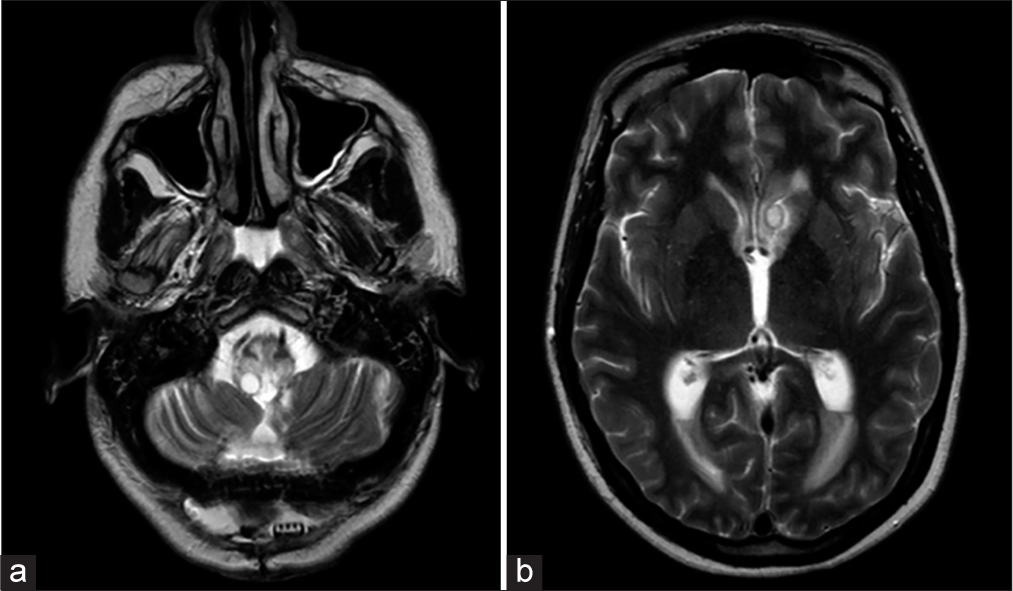
One-month later, the patient returned with a complaint of persistent postoperative pain, and a new area of swelling underlying the prior suboccipital incision for the past 4 days. She also endorsed progressive diplopia, blurry vision, and headaches while bending forward. On physical exam, there was a 1-cm erythematous fluctuant granulomatous nodule without frank discharge or CSF leak. The patient had a deconjugated gaze and inability to abduct the right eye and bilateral horizontal nystagmus without any additional lower cranial nerve dysfunction. MRI brain with contrast showed increased nodular leptomeningeal enhancement, and the interval development of multiple peripherally enhancing lesions throughout the brain parenchyma, the largest of which measured 1.4 cm within the left caudate [
Figure 4:
Magnetic resonance imaging (MRI) brain w/wo contrast – images obtained at time of most recent admission (a) axial T2 MRI w/o contrast, (b) axial T1 MRI w/contrast, and (c) axial T1 MRI w/contrast. Demonstrate a persistent pseudomeningocele with new parenchymal involvement and progression of leptomeningeal enhancement.
Several hours into her admission, the patient developed symptoms of acute hydrocephalus, having projectile emesis, and became obtunded. An emergent CT-head was obtained which confirmed acute hydrocephalus, and the patient was transferred to the neurological intensive care unit (ICU), where the VPS was explanted at bedside and a ventriculostomy catheter was soft passed through the prior right frontal burr hole until CSF egress was obtained. Despite the drain placement and adequate CSF drainage overnight, the following morning her clinical status continued to decline necessitating emergent intubation. Afterward, the patient was taken emergently to the operating room for suboccipital craniotomy and posterior fossa exploration.
Intraoperatively, there was no gross purulence encountered. Tissue obtained shows cerebellar parenchyma with epidermoid cyst, chronic inflammation, necrosis, granulation tissue, and foreign body reaction with degenerating keratin debris [
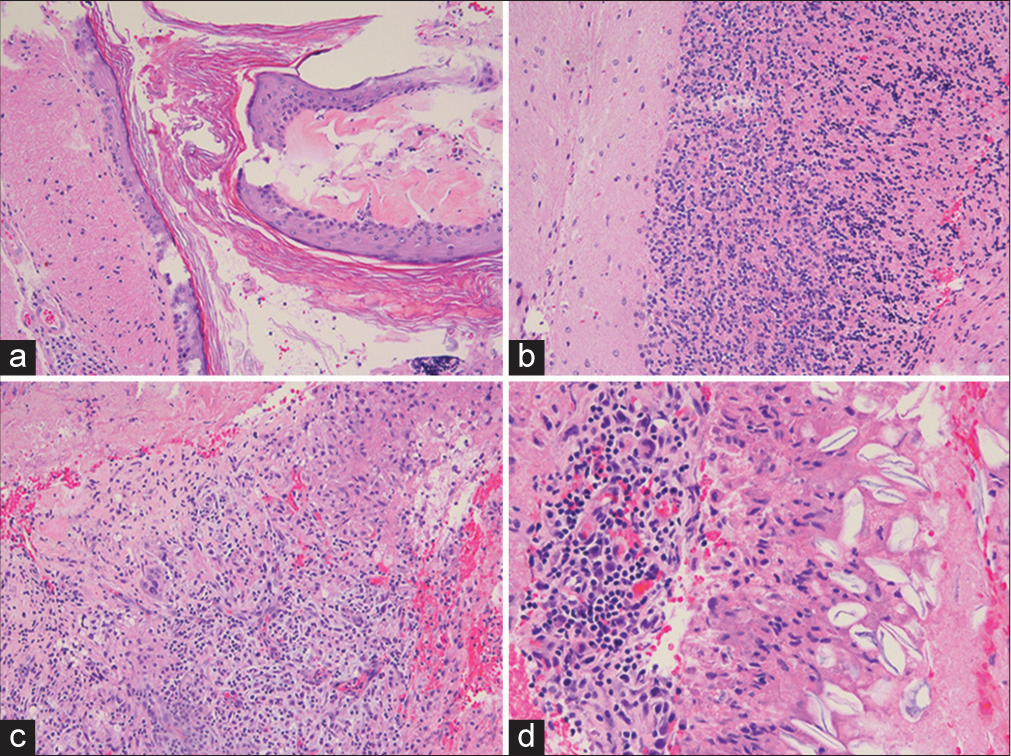
Figure 5:
Histopathological findings – (a) cyst lined by squamous epithelium with granular layer containing laminated keratin, consistent with epidermoid cyst. (b) Adjacent relatively uninvolved cerebellar parenchyma. (c) Necrosis with inflammation, granulation tissue, and giant cells. (d) Degenerating keratinocytes with inflammation and histiocytic reaction.
Ultimately, following multidisciplinary discussions between infectious disease, immunology, rheumatology, and neurology for atypical clinical course, the patient was started on high-dose prednisone (60 mg) for severe chemical meningitis.
The patient’s clinical examination slowly improved to the point of being able to follow simple one-step commands. Subsequent CSF analysis began showing improvement in profile (WBC 4/mm2, 45% neutrophils, 25% lymphocytes, 88 mg/dL glucose, and 9 mg/dL protein) and the leptomeningeal enhancement was no longer identified on further follow-up imaging. Throughout the remainder of her hospitalization, her mentation continued to improve. The VPS was replaced before her discharge to a neurorehabilitation facility, where she completed a long course of therapy. The patient is now 24 months postoperative form her second resection, with ataxic gait and partial 6th and 7th nerve palsies bilaterally.
DISCUSSION
Aseptic meningitis
Typically following epidermoid resection, resultant postoperative aseptic meningitis is thought to arise from the release of breakdown products, mainly keratin and cholesterol, from either the spontaneous leakage or surgical release of epidermoid contents into subarachnoid spaces leading to the inflammatory reaction.[
Characteristic symptoms of aseptic meningitis include headaches, vomiting, fevers, meningismus, and cognitive impairment.[
Hydrocephalus can often be associated with aseptic meningitis. The occurrence of aseptic meningitis is the result of epidermoid cyst contents entering the subarachnoid space which results in a cascade of inflammatory changes.[
The treatment for patients with aseptic meningitis, with or without hydrocephalus, involves the initial use of antibiotic therapy until infectious meningitis etiologies are ruled out, at which point they should be discontinued and systemic steroid treatment initiated, along with CSF diversion if clinical signs and symptoms of hydrocephalus are present.[
Inflammatory mediators
The specific biochemical inflammatory cascade driven by the breakdown products such as keratin in epidermoid cysts that result in aseptic meningitis has not been thoroughly investigated. However, a study by Cuff et al. found interleukin-6 (IL-6) to be elevated in patients with either an infectious or noninfectious cause of meningitis and lower levels of IL-17 in noninfectious meningitis patients.[
Steroids are known to be responsible for the alteration of inflammatory gene transcription. In animal models, it has been shown that dexamethasone reduces concentrations of IL-1β and TNF-α, especially in bacterial meningitis.[
CONCLUSION
To the author’s knowledge, this is the first reported case of such a protracted course of aseptic meningitis requiring a lengthy critical care period in the months following the resection of a posterior fossa epidermoid cyst. While aseptic meningitis following resection of epidermoid cysts is not a rare phenomenon, the clinical course and severity of the inflammatory reaction in this patient is quite unique. The patient’s clinical course was presumed to be associated with the above-described hyperreactive inflammatory response to residual postoperative keratin debris that resulted in this delayed episode of acute hydrocephalus and severe inflammatory changes throughout the brain. No infectious etiology was ever identified during the patient’s extensive clinical course. The authors report the above-illustrated case in the hopes of increasing the neurosurgeon’s awareness to the possibility of this rare but severe presentation as there are currently no criteria to identify those patients at risk. Today, the patient lives independently.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol. 2006. 148: 245-54
2. Berger MS, Wilson CB. Epidermoid cysts of the posterior fossa. J Neurosurg. 1985. 62: 214-9
3. Blitz SE, Bernstock JD, Dmytriw AA, Ditoro DF, Kappel AD, Gormley WB. Ruptured suprasellar dermoid cyst treated with lumbar drain to prevent postoperative hydrocephalus: Case report and focused review of literature. Front Surg. 2021. 8: 714771
4. Brown EM, de Louvois J, Bayston R, Lees PD, Pople IK. The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Br J Neurosurg. 2000. 14: 7-12
5. Cantu RC, Ojemann RG. Glucosteroid treatment of keratin meningitis following removal of a fourth ventricle epidermoid tumour. J Neurol Neurosurg Psychiatry. 1968. 31: 73-5
6. Cuff SM, Merola JP, Twohig JP, Eberl M, Gray WP. Toll-like receptor linked cytokine profiles in cerebrospinal fluid discriminate neurological infection from sterile inflammation. Brain Commun. 2020. 2: fcaa218
7. Davies EG, Gibb D, Kroll S, Levin M, Rudd P, Tarlow MJ. Should we use dexamethasone in meningitis?. Arch Dis Child. 1992. 67: 1398-401
8. Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D. Complications of posterior cranial fossa surgery-an institutional experience of 500 patients. Surg Neurol. 2009. 72: 369-75
9. Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001. 32: 179-85
10. Hillier CE, Stevens AP, Thomas F, Vafidis J, Hatfield R. Aseptic meningitis after posterior fossa surgery treated by pseudomeningocele closure. J Neurol Neurosurg Psychiatry. 2000. 68: 218-9
11. Kaufman HH, Carmel PW. Aseptic meningitis and hydrocephalus after posterior fossa surgery. Acta Neurochir1978;. 44: 179-96
12. Marshman LA, Benjamin JC, Chawda SJ, David KM. Acute obstructive hydrocephalus associated with infratentorial subdural hygromas complicating Chiari malformation Type I decompression. Report of two cases and literature review. J Neurosurg. 2005. 103: 752-5
13. Mehendale NH, Samy RN, Roland PS. Management of pseudomeningocele following neurotologic procedures. Otolaryngol Head Neck Surg. 2004. 131: 253-62
14. Munakomi S, Bhattarai B, Chaudhary P. Case report: Acute obstructive hydrocephalus associated with infratentorial extra-axial fluid collection following foramen magnum decompression and durotomy for Chiari malformation Type I. F1000Res. 2016. 5: 33
15. Nadraga A, Khomyn O. Cerebrospinal fluid inflammatory markers in children with aseptic meningitis. Curr Issues Pharm Med Sci. 2020. 33: 6-9
16. Ng WP, Liew BS, Gee TS, Azmin KR. Posterior fossa intradiploic epidermoid cyst: A case report. Int Med J Malays. 2015. 14: 67-70
17. Pirouzmand F, Tator CH, Rutka J. Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery. 2001. 48: 1246-53 discussion 1253-4
18. Rutherford SA, Leach PA, King AT. Early recurrence of an intracranial epidermoid cyst due to low-grade infection: Case report. Skull Base. 2006. 16: 109-16
19. Schwartz JF, Balentine JD. Recurrent meningitis due to an intracranial epidermoid. Neurology. 1978. 28: 124-9
20. Spinato G, Gaudioso P, Falcioni M, Da Mosto MC, Cocuzza S, Maniaci A. Giant epidermoid cyst of posterior fossaour experience and literature review. Dose Response. 2021. 19: 15593258211002061
21. Tanabe K, Matsushima-Nishiwaki R, Yamaguchi S, Iida H, Dohi S, Kozawa O. Mechanisms of tumor necrosis factor-a-induced interleukin-6 synthesis in glioma cells. J Neuroinflammation. 2010. 7: 16
22. Xuzhi H, Xuhui W, Minhui X, Hong L, Lunshan X. Diagnosis and treatment of postoperative aseptic meningitis. Sci Res Essays. 2011. 6: 2221-4
23. Yamakawa K, Shitara N, Genka S, Manaka S, Takakura K. Clinical course and surgical prognosis of 33 cases of intracranial epidermoid tumors. Neurosurgery. 1989. 24: 568-73
24. Yasargil MG, Abernathe CD, Sarioglu AC. Microneurosurgical treatment of intracranial dermoid and epidermoid tumors. Neurosurgery. 1989. 24: 561-7