- Department Neurosurgery, University of Chicago, Chicago, Illinois, United States.
- Department Radiology, University of Chicago, Chicago, Illinois, United States.
- Department Neurology, University of Chicago, Chicago, Illinois, United States.
Correspondence Address:
Andrew Platt
Department Neurology, University of Chicago, Chicago, Illinois, United States.
DOI:10.25259/SNI_905_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Andrew Platt1, John Collins2, Edwin Ramos1, Fernando D. Goldenberg3. Pseudosubarachnoid hemorrhage: A systematic review of causes, diagnostic modalities, and outcomes in patients who present with pseudosubarachnoid hemorrhage. 20-Jan-2021;12:29
How to cite this URL: Andrew Platt1, John Collins2, Edwin Ramos1, Fernando D. Goldenberg3. Pseudosubarachnoid hemorrhage: A systematic review of causes, diagnostic modalities, and outcomes in patients who present with pseudosubarachnoid hemorrhage. 20-Jan-2021;12:29. Available from: https://surgicalneurologyint.com/surgicalint-articles/10530/
Abstract
Background: Patients with computed tomography (CT) findings consistent with subarachnoid hemorrhage without evidence of hemorrhage following autopsy or cerebrospinal fluid testing are termed to have pseudosubarachnoid hemorrhage (pSAH).
Methods: A systematic review of literature was conducted based on the preferred reporting items for systematic reviews and meta-analysis statement. Studies were evaluated for associated cause of pSAH, imaging modality used in assessment, method of confirmatory testing, and clinical outcome.
Results: Fifty studies were included in qualitative analysis including 197 cases of pSAH. Systematic review revealed 23 studies including 110 patients with pSAH attributed to hypoxic-ischemic brain injury following cardiac arrest. Three studies were included in meta-analysis that quantitatively analyzed differences in CT densities in patients with pSAH and true subarachnoid hemorrhage (true SAH). A random effects model meta-analysis showed a statistically significant decrease in densities in the Sylvian fissure in patients with pSAH compared to true SAH and a statistically significant decrease in densities in adjacent parenchyma in patients with pSAH compared to true SAH. Systematic review further revealed 32 patients with pSAH associated with spontaneous intracranial hypotension, 11 patients with pSAH related to infectious etiologies, 15 patients with pSAH associated with subdural hemorrhage, 20 cases of pSAH related to hyperhemoglobinemia, 2 cases related to valproate toxicity, and individual cases related to hyponatremia, diabetic ketoacidosis, sudden infant death syndrome, cerebellar infarction, and dialysis disequilibrium syndrome.
Conclusion: This study is the first systematic review of causes, diagnostic modalities, and outcomes in patients who present with pSAH. A diagnosis of pSAH may be considered following assessment of CT densities following cardiac arrest.
Keywords: Cardiac arrest, False subarachnoid hemorrhage, Pseudosubarachnoid hemorrhage, Systematic review
INTRODUCTION
Traumatic brain injury and aneurysmal rupture are the most common causes of subarachnoid hemorrhage; however, the differential diagnosis includes several etiologies. Computed tomography (CT) is the primary testing modality in the diagnosis of subarachnoid hemorrhage and has, with advances in technology, approached greater than 90% sensitivity and nearly 100% specificity in the diagnosis of aneurysmal subarachnoid hemorrhage.[
Several etiologies of pSAH have been described including cerebral edema related to hypoxic-ischemic brain injury, meningeal infection, cerebral/cerebellar infarction, subdural hemorrhage, spontaneous intracranial hypotension, hyperhemoglobinemia, and iatrogenic causes following intrathecal, intravenous, or intra-arterial contrast administration.[
Several case reports and case series have been written concerning pSAH, however, there have been no significant reviews. This study is a systematic review of causes, diagnostic modalities, and outcomes in patients who present with pSAH as well as a meta-analysis comparing CT imaging characteristics of patients with pSAH secondary to hypoxicischemic brain injury and true subarachnoid hemorrhage (true SAH).
MATERIALS AND METHODS
This study includes a systematic review of literature conducted based on the preferred reporting items for systematic reviews and meta-analysis statement.[
Case reports, case series, comparative studies including randomized controlled trials, prospective/retrospective cohort, and case–control studies were included in further analysis. Non-English publications, editorials, conference abstracts, errata, and book chapters were excluded. Only studies including patients with radiographic and clinical findings concerning for possible subarachnoid hemorrhage, that were later ruled nonsubarachnoid hemorrhage, were included in further analysis. Patients with true SAH of aneurysmal or nonaneurysmal origin were excluded from the study. Cases of patients with pSAH following iatrogenic administration of contrast including patients with postprocedural contrast neurotoxicity or gadolinium encephalopathy were excluded from the study. Studies that were not available to be read in entirety were excluded from the study. Studies were evaluated for associated cause of pSAH, imaging modality used in assessment, method of confirmatory testing, and clinical outcome.
A standardized mean difference and 95% confidence interval were calculated for continuous outcomes that were then pooled by random effects model meta-analysis. All statistical tests were performed using RevMan 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). An I2 test was performed for each comparison to test statistical heterogeneity with I2 values exceeding 25%, 50%, and 75% indicating a low, moderate, and high degree of heterogeneity, respectively. For all meta-analyses, outcomes were pooled with weights calculated by the inverse-variance method. P < 0.5 was used to assess statistical significance.
RESULTS
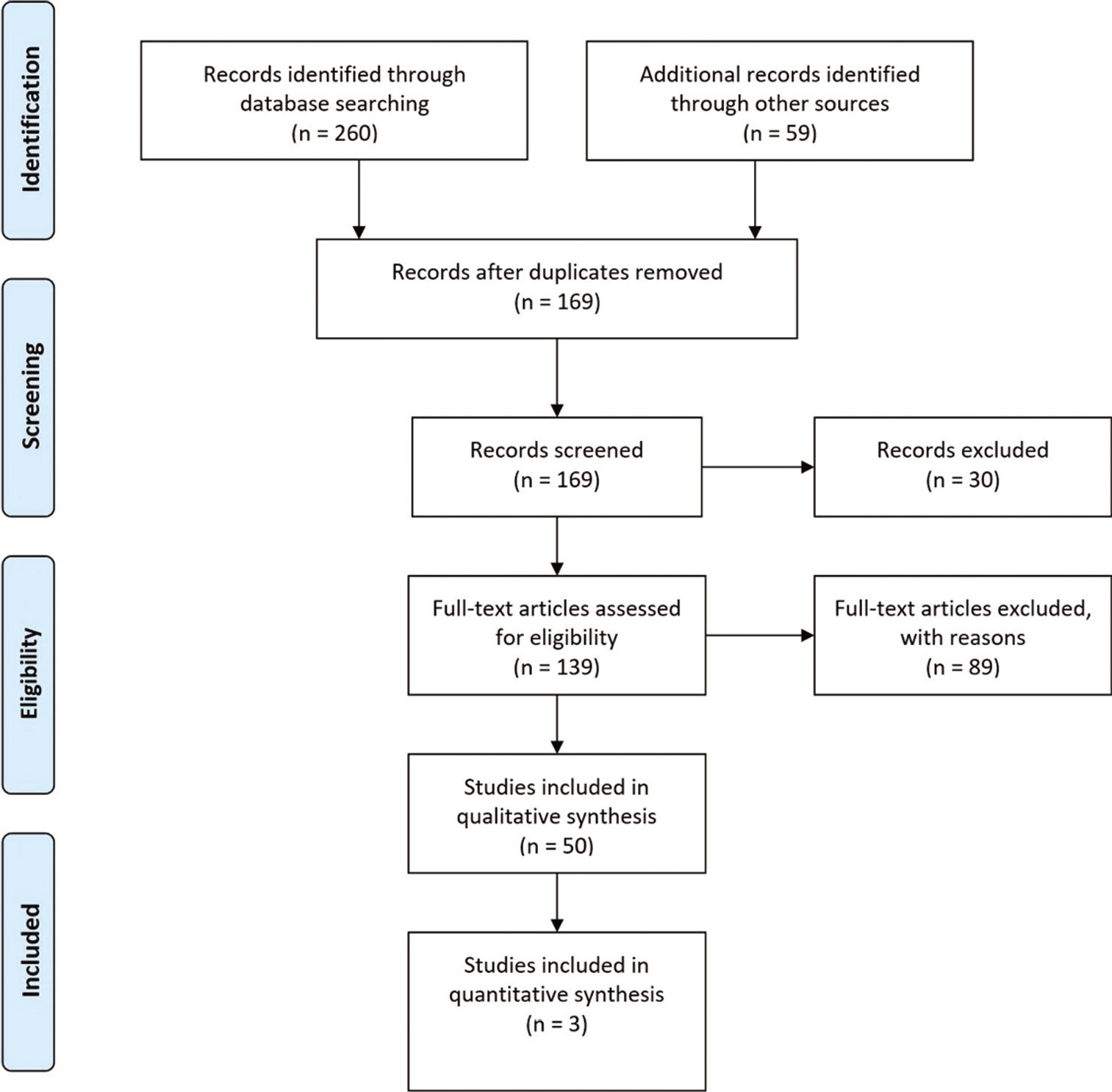
In total, 169 abstracts were reviewed of which 30 were excluded. Several studies were excluded as full manuscripts were unable to be obtained. One hundred and thirty-nine full text articles were assessed of which 89 were excluded. Several studies of iatrogenic pSAH were excluded. [
excluded for not specifying associated causes of pSAH, for including only patients with postmortem radiography, or for including patients without pSAH.[
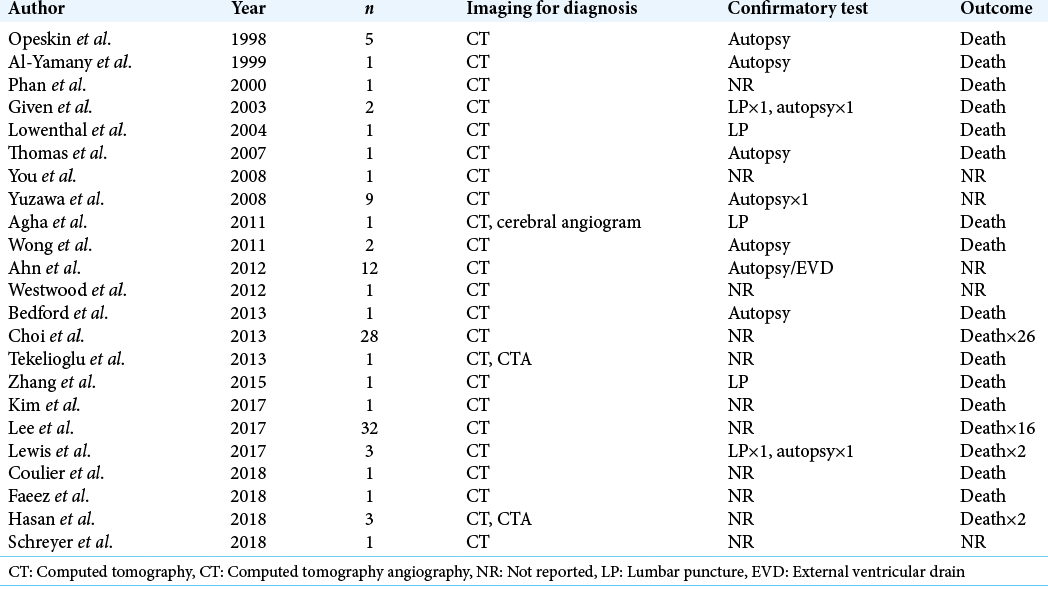
Systematic review revealed 23 studies of patients with pSAH following hypoxic-ischemic injury. Two individual patients were excluded from included studies for having initial CT scans following cardiac catheterization.[
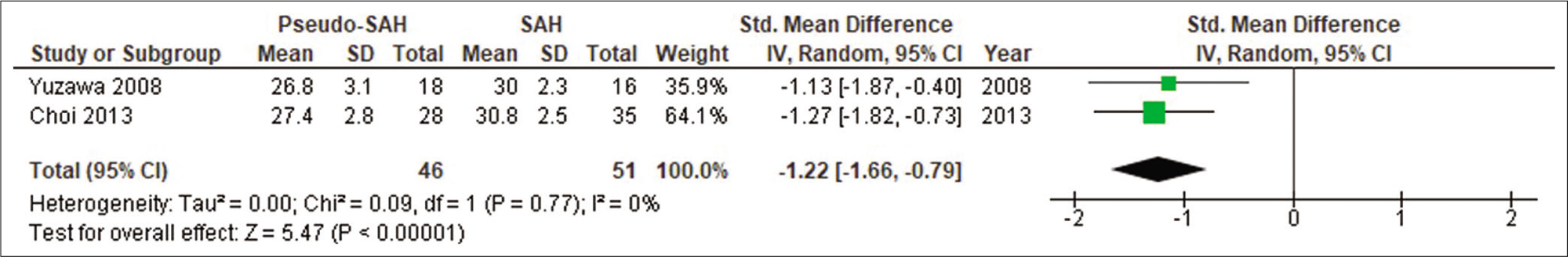
Yuzawa et al. measured densities bilaterally and included 18 hemispheres in the pSAH group for both HDA and parenchymal measurements, 14 hemispheres in the true SAH group for HDA measurements, and 16 hemispheres in the true SAH group for parenchymal measurements.[
Ahn et al. also measured densities bilaterally and included 12 patients (24 hemispheres) in each pSAH and true SAH group. They defined patients as pSAH following negative confirmatory testing (LP or autopsy) or through clinical characteristics similar to Yuzawa et al. Patients defined clinically and those defined through confirmatory testing did not have a statistically significant difference in averaged measured densities.[
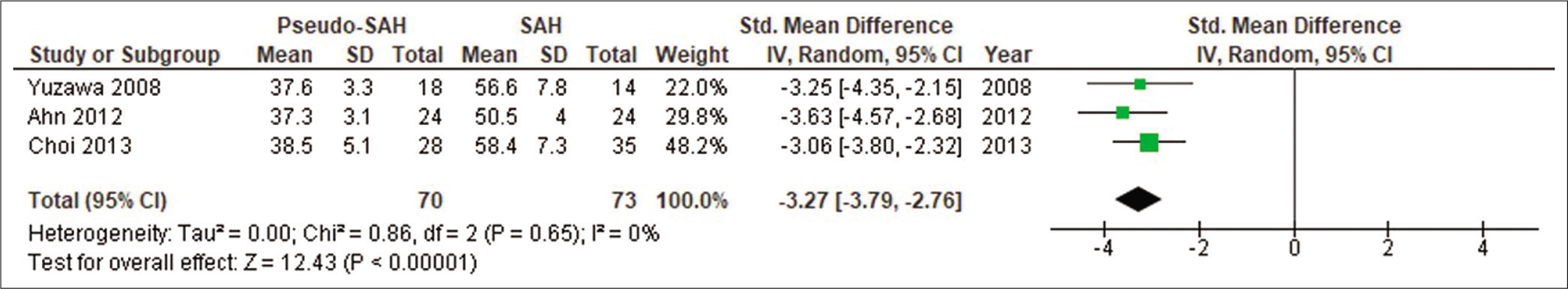
In comparing densities in HDA, 70 hemispheres were included in the pSAH group and 73 hemispheres were included in the true SAH group. Mean densities ranged from 37.3 to 38.5 HU in the pSAH group and 50.5 to 58.4 HU in the true SAH group. A random effects model meta-analysis was carried out which showed a statistically significant decrease in densities in HDA in patients with pSAH compared to true SAH (SMD –3.27; 95% CI –3.79, –2.76; P < 0.00001; I2 = 0) [
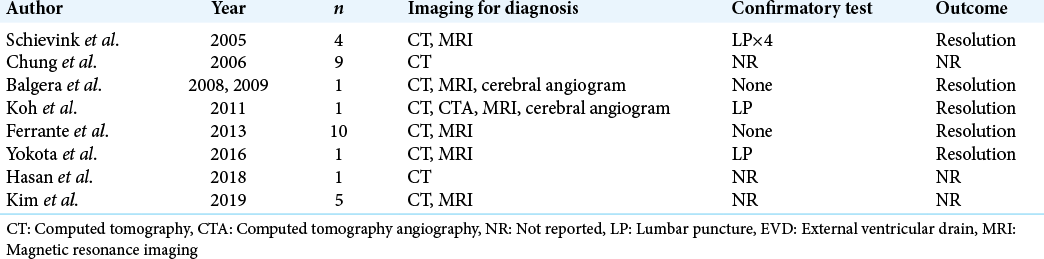
Systematic review revealed nine studies of 32 patients with pSAH associated with spontaneous intracranial hypotension [
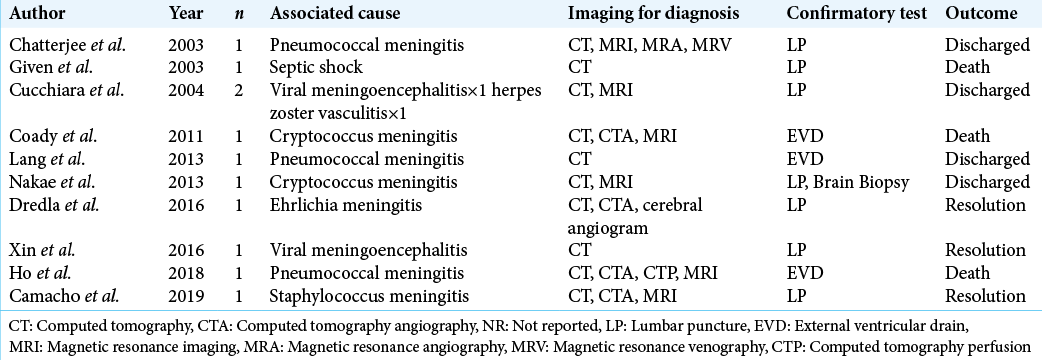
Eleven patients with pSAH related to infectious etiologies were discovered across 10 manuscripts [
Systematic review further revealed 15 cases of pSAH associated with subdural hemorrhages.[
DISCUSSION
Early recognition of aneurysmal subarachnoid hemorrhage following presentation is essential to improving patient outcomes.[
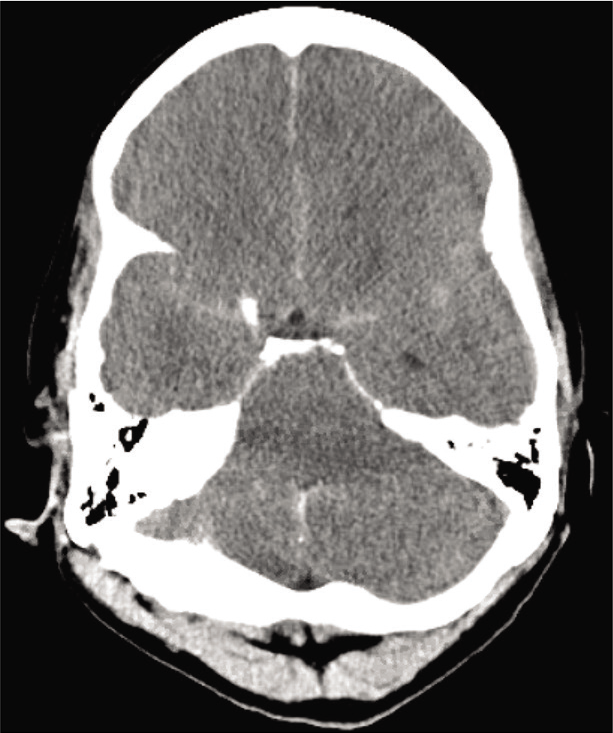
All of the 197 cases of pSAH revealed in systematic review had CT scans that revealed HDA within the subarachnoid space. Compared with patients with true SAH, meta-analysis has shown patients with pSAH, associated with hypoxicischemic injury, to have significantly decreased densities in HDA and adjacent parenchyma [
The confirmatory test of choice in patients with pSAH remains CSF sampling through LP or EVD. Its utility is not only in ruling out aneurysmal subarachnoid hemorrhage but also in potentially identifying the cause of pSAH. CSF sampling is especially important in cases of central nervous system infection as it can be used to rule out subarachnoid hemorrhage and identify the causative pathogen. Performing a LP to rule out aneurysmal subarachnoid hemorrhage is also controversial, especially regarding the interpretation of CSF results and xanthochromia.[
Individual laboratory testing tailored to the presumed cause of pSAH has also been shown to be effective. In cases of hyperhemoglobinemia, the previous studies have found hematocrit levels to range from 62.3 to 74.8%.[
Outcomes following pSAH vary greatly according to the underlying etiology. In studies reporting outcomes of patients with pSAH following hypoxic-ischemic injury, the mortality rate was 77%, however, in studies reporting outcomes of patients with spontaneous intracranial hypotension, the mortality rate was 0% with 100% of patients having complete resolution of symptoms following treatment. In studies reporting outcomes of patients with pSAH related to central nervous system infection, the mortality rate was 27%. In patients who present following cardiac arrest, pSAH has further been identified as a significant predictor of increased in-hospital mortality and poor neurologic outcome compared to patients who present following cardiac arrest without evidence of pSAH.[
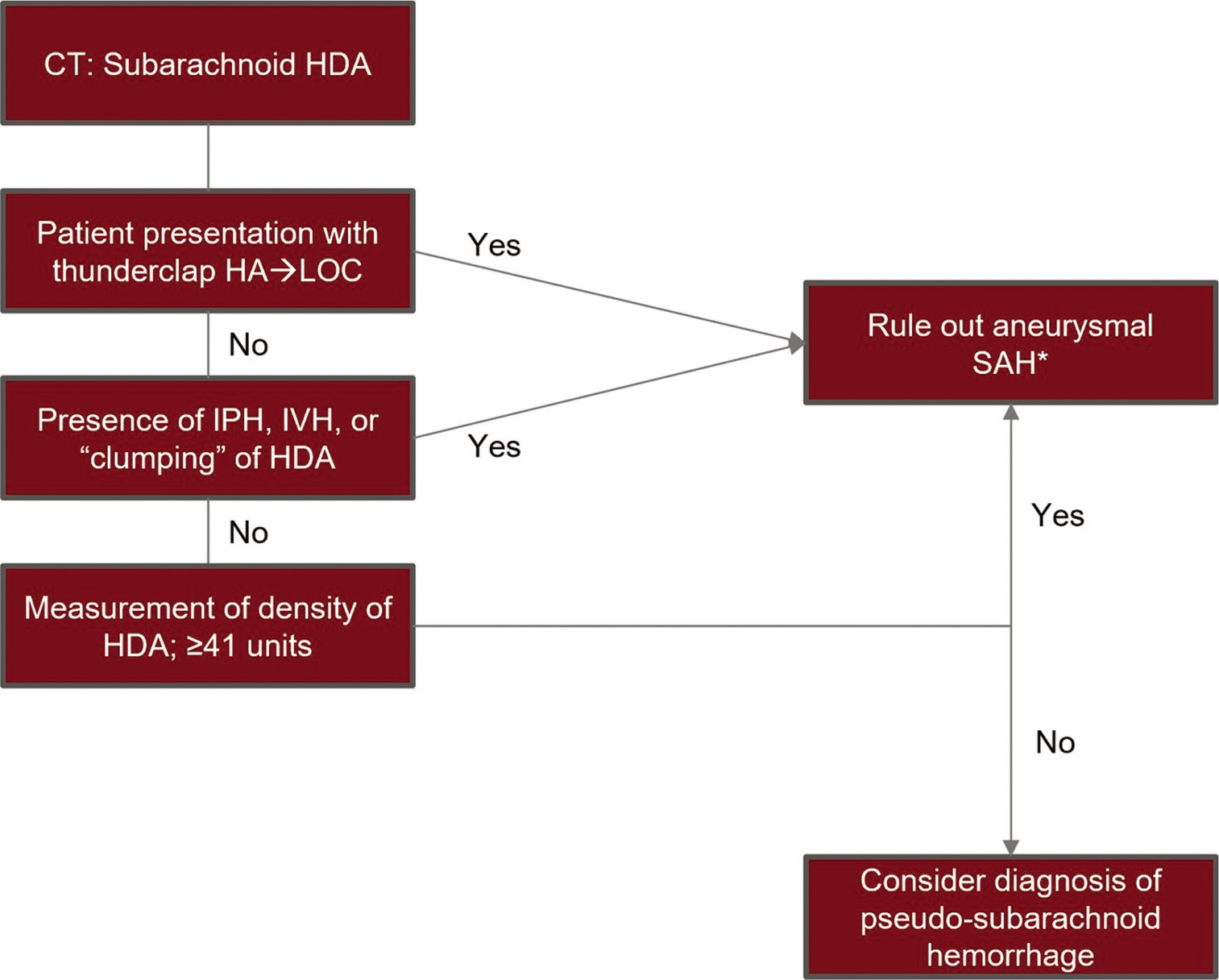
Given the results of this systematic review and meta-analysis, we have proposed the following theoretical diagnostic protocol in patients with CT findings of HDAs within the subarachnoid space following resuscitation from cardiac arrest [
Figure 5:
A flowchart of a theoretical diagnostic protocol to differentiate cases of pseudosubarachnoid hemorrhage from aneurysmal subarachnoid hemorrhage in patients who present following cardiac arrest with high-density areas on computed tomography. *Ruling out subarachnoid hemorrhage to be done per individual hospital policy which may include further vascular imaging or confirmatory testing, HAD: High-density areas, HA: Headache, LOC: Loss of consciousness, IPH: Intraparenchymal hemorrhage, IVH: Intraventricular hemorrhage.
Limitations
There are several limitations to this study. Following systematic review, 50 studies were included in qualitative analysis, however, only three studies met inclusion criteria for quantitative analysis. Although all studies were directly comparative in nature, studies defined patients as pSAH mostly from clinical presentation and radiologic findings, not through confirmatory testing. This study was also limited by heterogeneity within the true SAH group, and low patient samples and incomplete reported data.
CONCLUSION
This study, to the best of our knowledge, is the first systematic review of causes, diagnostic modalities, and outcomes in patients who present with pSAH. This study is also the only meta-analysis comparing CT imaging characteristics of patients with pSAH related to hypoxic-ischemic brain injury and true SAH. Although aneurysmal subarachnoid hemorrhage must always remain at the top of a differential when a patient presents with HDAs within the subarachnoid space, a diagnosis of pSAH may be considered following assessment of CT densities in a patient that presents following cardiac arrest. Furthermore, patient history is essential to diagnose other causes of pSAH and can be aided by targeted diagnostic tests including MRI to assess for cases of spontaneous intracranial hypotension, CSF analysis to assess for central nervous system infection, and specific laboratory tests including hemoglobin/hematocrit to assess for hyperhemoglobinemia and valproate levels to assess for valproate toxicity.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Agha A, Al-Hakami M. A case report of pseudo-subarachnoid hemorrhage. Maedica (Bucur). 2011. 6: 210-2
2. Ahn JH, Choi SC, Jung YS, Min YG. Clinical characteristics of patients with pseudo-subarachnoid haemorrhage who were successfully resuscitated from out-of-hospital cardiopulmonary arrest. Hong Kong J Emerg Med. 2012. 19: 85-91
3. Al-Yamany M, Deck J, Bernstein M. Pseudo-subarachnoid hemorrhage: A rare neuroimaging pitfall. Can J Neurol Sci. 1999. 26: 57-9
4. Arlt S, Cepek L, Rustenbeck HH, Prange H, Reimers CD. Gadolinium encephalopathy due to accidental intrathecal administration of gadopentetate dimeglumine. J Neurol. 2007. 254: 810-2
5. Atsumi H, Sorimachi T, Nonaka Y, Matsumae M. Basal cistern effacement and pseudo-subarachnoid hemorrhage on computed tomography images of chronic subdural hematoma. World Neurosurg. 2019. 132: e109-15
6. Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol. 1998. 28: 222-5
7. Balgera R, Rigamonti A, Agostoni E. Images from headache: Spontaneous intracranial hypotension presenting as pseudo subarachnoid hemorrhage. Headache. 2008. 48: 614-5
8. Balgera R, Rigamonti A, Sozzi G, Agostoni E. An atypical case of spontaneous intracranial hypotension. Neurol Sci. 2009. 30: 71-3
9. Barton BR, Prabhakaran S, Lopes DK, Lee VH. Pseudo-subarachnoid hemorrhage in cerebellar infarction. Neurocrit Care. 2007. 7: 172-4
10. Bedford P, O’Donnell C. Pseudosubarachnoid hemorrhage on clinical computed tomography: The forensic implications of incorrect diagnosis. Am J Forensic Med Pathol. 2013. 34: e4-6
11. Blok KM, Rinkel GJ, Majoie CB, Hendrikse J, Braaksma M, Tijssen CC. CT within 6 hours of headache onset to rule out subarachnoid hemorrhage in nonacademic hospitals. Neurology. 2015. 84: 1927-32
12. Camacho MA, Druck J, Musi M. Subarachnoid mirage: A case of pseudosubarachnoid hemorrhage. Ann Emerg Med. 2019. 73: 130-2
13. Chatterjee T, Gowardman JR, Goh TD. Pneumococcal meningitis masquerading as subarachnoid haemorrhage. Med J Aust. 2003. 178: 505-7
14. Choi KS, Won YD, Yi HJ, Lim TH, Lee YJ, Chun HJ. Therapeutic and prognostic implications of subarachnoid hemorrhage in patients who suffered cardiopulmonary arrest and underwent cardiopulmonary resuscitation during an emergency room stay. Clin Neurol Neurosurg. 2013. 115: 2088-93
15. Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC. Subdural hematoma in spontaneous CSF hypovolemia. Neurology. 2006. 67: 1088-9
16. Coady G, Brewer M, Maillloux P. Pseudosubarachnoid hemorrhage in a 42-year-old male with meningitis. Radiol Case Rep. 2011. 6: 470
17. Coulier B. Pseudo-subarachnoid Hemorrhage. J Belg Soc Radiol. 2018. 102: 32
18. Cucchiara B, Sinson G, Kasner SE, Chalela JA. Pseudo-subarachnoid hemorrhage: Report of three cases and review of the literature. Neurocrit Care. 2004. 1: 371-4
19. Dadpour B, Abbaspour H, Pourzahed A, Moghadam AB. Pseudo-subarachnoid hemorrhage and optic neuritis in an 18-year-old girl with sodium valproate overdose. J Res Med Sci. 2016. 21: 43
20. Dredla B, Freeman WD. Ehrlichia meningitis mimicking aneurysmal subarachnoid hemorrhage: A case study for medical decision-making heuristics. Neurohospitalist. 2016. 6: 76-9
21. Eckel TS, Breiter SN, Monsein LH. Subarachnoid contrast enhancement after spinal angiography mimicking diffuse subarachnoid hemorrhage. AJR Am J Roentgenol. 1998. 170: 503-5
22. Ferrante E, Regna-Gladin C, Arpino I, Rubino F, Porrinis L, Ferrante MM. Pseudo-subarachnoid hemorrhage: A potential imaging pitfall associated with spontaneous intracranial hypotension. Clin Neurol Neurosurg. 2013. 115: 2324-8
23. Given CA, Burdette JH, Elster AD, Williams DW. Pseudo-subarachnoid hemorrhage: A potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003. 24: 254-6
24. Hasan TF, Duarte W, Akinduro OO, Goldstein ED, Hurst R, Haranhalli N. Nonaneurysmal “pseudo-subarachnoid hemorrhage” computed tomography patterns: Challenges in an acute decision-making heuristics. J Stroke Cerebrovasc Dis. 2018. 27: 2319-26
25. Ho AL, Sussman ES, Pendharkar AV, Iv M, Hirsch KG, Fischbein NJ. Practical pearl: Use of MRI to differentiate pseudo-subarachnoid hemorrhage from true subarachnoid hemorrhage. Neurocrit Care. 2018. 29: 113-8
26. Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med. 2012. 42: e109-11
27. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968. 28: 14-20
28. Javedan SP, Marciano F. Pseudo-enhancement from polycythemia. Neurology. 2004. 62: 150
29. Kim JH, Roh H, Yoon WK, Kwon TH, Chong K, Hwang SY. Clinical features of patients with spontaneous intracranial hypotension complicated with bilateral subdural fluid collections. Headache. 2019. 59: 775-86
30. Kim JM, Eom TH. The pseudosubarachnoid sign: Clinical implications of subarachnoid hemorrhage misdiagnosis. Pediatr Emerg Care. 2017. 33: e170-1
31. Koh E, Huang SH, Lai YJ, Hong CT. Spontaneous intracranial hypotension presenting as pseudo-subarachnoid hemorrhage on CT scan. J Clin Neurosci. 2011. 18: 1264-5
32. Lang JL, Leach PL, Emelifeonwu JA, Bukhari S. Meningitis presenting as spontaneous subarachnoid haemorrhage (pseudo-subarachnoid haemorrhage). Eur J Emerg Med. 2013. 20: 140-1
33. Lee BK, Kim YJ, Ryoo SM, Kim SJ, Lee DH, Jeung KW. “Pseudo-subarachnoid hemorrhage sign” on early brain computed tomography in out-of-hospital cardiac arrest survivors receiving targeted temperature management. J Crit Care. 2017. 40: 36-40
34. Lewis OD, Afreen S, Folaranmi S, Fidelia-Lambert M, Poddar V, Thomas A. Anoxic brain injury presenting as pseudosubarachnoid hemorrhage in the medical intensive care unit. Case Rep Crit Care. 2017. 2017: 9071482
35. Li H, Ayinuer A, Huang J. Correlation between hyperhemoglobinemia and pseudosubarachnoid hemorrhage. Clin Imaging. 2020. 59: 8-12
36. Li L, Gao FQ, Zhang B, Luo BN, Yang ZY, Zhao J. Overdosage of intrathecal gadolinium and neurological response. Clin Radiol. 2008. 63: 1063-8
37. Lin CY, Lai PH, Fu JH, Wang PC, Pan HB. Pseudo-subarachnoid hemorrhage: A potential imaging pitfall. Can Assoc Radiol J. 2014. 65: 225-31
38. Long B, Koyfman A, Runyon MS. Subarachnoid hemorrhage: Updates in diagnosis and management. Emerg Med Clin North Am. 2017. 35: 803-24
39. Lowenthal A, Maimon N, Waldman S, Almog Y. Sub-arachnoid hemorrhage following cardiopulmonary resuscitation. Resuscitation. 2004. 63: 221-3
40. Md Noh MS, Rashid AM. Development of pseudo-subarachnoid hemorrhage secondary to hypoxic-ischemic injury due to bleeding pulmonary arterio-venous malformation. BMC Neurol. 2018. 18: 157
41. Merchut MP, Richie B. Transient visuospatial disorder from angiographic contrast. Arch Neurol. 2002. 59: 851-4
42. Min YG, Tse ML. Image in toxicology: Pseudo-subarachnoid hemorrhage in a case of severe valproic acid poisoning. Clin Toxicol (Phila). 2011. 49: 699-700
43. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009. 6: e1000097
44. Nakae Y, Kudo Y, Yamamoto R, Johkura K. Pseudo-subarachnoid hemorrhage in cryptococcal meningitis: MRI findings and pathological study. Neurol Sci. 2013. 34: 2227-9
45. Oh CH, An SD, Choi SH, Ji GY. Contrast mimicking a subarachnoid hemorrhage after lumbar percutaneous epidural neuroplasty: A case report. J Med Case Rep. 2013. 7: 88
46. Opeskin K, Silberstein M. False positive diagnosis of subarachnoid haemorrhage on computed tomography scan. J Clin Neurosci. 1998. 5: 382-6
47. Patzig M, Laub C, Janssen H, Ertl L, Fesl G. Pseudo-subarachnoid haemorrhage due to chronic hypoxaemia: Case report and review of the literature. BMC Neurol. 2014. 14: 219
48. Perry JJ, Stiell IG, Sivilotti ML, Bullard MJ, Emond M, Symington C. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: Prospective cohort study. BMJ. 2011. 343: d4277
49. Phan TG, Wijdicks EF, Worrell GA, Fulgham JR. False subarachnoid hemorrhage in anoxic encephalopathy with brain swelling. J Neuroimaging. 2000. 10: 236-8
50. Potsi S, Chourmouzi D, Moumtzouoglou A, Nikiforaki A, Gkouvas K, Drevelegas A. Transient contrast encephalopathy after carotid angiography mimicking diffuse subarachnoid haemorrhage. Neurol Sci. 2012. 33: 445-8
51. Rabinstein AA. Pseudosubarachnoid haemorrhage in subdural haematoma. J Neurol Neurosurg Psychiatry. 2003. 74: 1131-2
52. Samardzic D, Thamburaj K. Magnetic resonance characteristics and susceptibility weighted imaging of the brain in gadolinium encephalopathy. J Neuroimaging. 2015. 25: 136-9
53. Sasaki Y, Ishii K, Ishii I. Myelography-associated pseudo-subarachnoid hemorrhage. Vis J Emerg Med. 2016. 5: 25-6
54. Schievink WI, Maya MM, Tourje J, Moser FG. Pseudo-subarachnoid hemorrhage: A CT-finding in spontaneous intracranial hypotension. Neurology. 2005. 65: 135-7
55. Schievink WI, Wijdicks EF, Meyer FB, Sonntag VK. Spontaneous intracranial hypotension mimicking aneurysmal subarachnoid hemorrhage. Neurosurgery. 2001. 48: 513-6
56. Schreyer KE, Surapaneni K, Sammon M. Pseudo-subarachnoid hemorrhage after cardiac arrest. Clin Pract Cases Emerg Med. 2018. 2: 95-6
57. Shirota G, Gonoi W, Ikemura M, Ishida M, Shintani Y, Abe H. The pseudo-SAH sign: An imaging pitfall in postmortem computed tomography. Int J Legal Med. 2017. 131: 1647-53
58. Son D, Kim Y, Kim C, Lee S. Pseudo-subarachnoid hemorrhage; chronic subdural hematoma with an unruptured aneurysm mistaken for subarachnoid hemorrhage. Korean J Neurotrauma. 2019. 15: 28-33
59. Spiegel SM, Fox AJ, Vinuela F, Pelz DM. Increased density of tentorium and falx: A false positive CT sign of subarachnoid hemorrhage. Can Assoc Radiol J. 1986. 37: 243-7
60. Thomas GL, Stachowski ER. Pseudosubarachnoid haemorrhage on CT brain scan: An unusual presentation of diffuse hypoxic brain injury. Intensive Care Med. 2007. 33: 2038-40
61. Tsai WC, Chen JC, Tsao YT. Pseudosubarachnoid hemorrhage: An ominous sign in dialysis disequilibrium syndrome. Am J Emerg Med. 2015. 33: 602.e3-4
62. Velden J, Milz P, Winkler F, Seelos K, Hamann GF. Nonionic contrast neurotoxicity after coronary angiography mimicking subarachnoid hemorrhage. Eur Neurol. 2003. 49: 249-51
63. Westwood AJ, Burns JD, Green DM. Teaching neuroimages: Pseudo-subarachnoid hemorrhage. Neurology. 2012. 78: e54
64. Wong LC, Schelvan C, Mitchell LA, Inwald DP. Computed tomography may demonstrate pseudosubarachnoid hemorrhage in diffuse cerebral edema after cardiorespiratory arrest. Pediatr Crit Care Med. 2011. 12: e208-10
65. Xin Y, Gao X, Ju X, Li A. Successful treatment with hyperbaric oxygen therapy for severe brain edema characterized by radiological appearance of pseudosubarachnoid hemorrhage in a child. Exp Ther Med. 2016. 12: 1625-7
66. Tekelioglu UY, Demirhan A, Akkaya A, Gurel K, Ocak T, Duran A. Pseudo-subarachnoid hemorrhage and death after a bee sting. Med Glas (Zenica). 2013. 10: 182-5
67. Yokota H, Yokoyama K, Nakase H. Spontaneous intracranial hypotension with pseudo-subarachnoid hemorrhage. Acta Neurol Belg. 2016. 116: 643-4
68. You JS, Park S, Park YS, Chung SP. Pseudo-subarachnoid hemorrhage. Am J Emerg Med. 2008. 26: 521.e1-2
69. Yuzawa H, Higano S, Mugikura S, Umetsu A, Murata T, Nakagawa A. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: Characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol. 2008. 29: 1544-9
70. Zhang J, Li Q, Zhang Z, Sun X. Pseudo-subarachnoid hemorrhage in a patient with hypoxic encephalopathy. Neurochirurgie. 2015. 61: 35-7