- Departments of Neurosurgery, General Hospital of Fortaleza, Avila Goulart, Fortaleza, Ceara, Brazil.
- Departments of Otorhinolaringology, General Hospital of Fortaleza, Avila Goulart, Fortaleza, Ceara, Brazil.
Correspondence Address:
Tiago Silva Holanda Ferreira
Departments of Neurosurgery, General Hospital of Fortaleza, Avila Goulart, Fortaleza, Ceara, Brazil.
DOI:10.25259/SNI_45_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Tiago Silva Holanda Ferreira1, Isnara Mara Freitas Pimentel2, Lucas Alverne Freitas de Albuquerque1, Jackson A. Gondim1. Pure endoscopic transsphenoidal treatment of skull base ameloblastoma with intracranial extension: Case report and literature review. 01-Aug-2020;11:228
How to cite this URL: Tiago Silva Holanda Ferreira1, Isnara Mara Freitas Pimentel2, Lucas Alverne Freitas de Albuquerque1, Jackson A. Gondim1. Pure endoscopic transsphenoidal treatment of skull base ameloblastoma with intracranial extension: Case report and literature review. 01-Aug-2020;11:228. Available from: https://surgicalneurologyint.com/surgicalint-articles/10182/
Abstract
Background: Ameloblastoma is a benign locally invasive lesion that represents 1% of all oral tumors. Epidemiological characteristics are variable in the literature. The most common origin sites are mandible and maxilla. Rarely presents metastasis, but the skull base, lymph nodes, and the lung are described as metastatic sites. Low recurrence rates were reported by the authors when surgical treatment achieved complete resection.
Case Description: A female patient, 19 years old presenting moderate headache associated with nausea, vomiting, left facial hypoesthesia, and low visual acuity. Resonance image showed a heterogeneous expansive solid formation in sphenoid bone and clivus with neoplastic aspect. Signs of dissemination due to contiguity and invasion of skull base structures, especially cavernous sinus and internal carotid artery, determining also compression of the brainstem. First, an endoscopic biopsy was performed with otorhinolaryngology service. The pathological study showed histological characteristics of ameloblastoma. After, the patient was submitted to endoscopic surgery for resection of tumor.
Conclusion: Ameloblastoma is a rare tumor with benign behavior and slow growing. It arises from odontogenic epithelium and accounts 1% of all oral tumors. The mandible and maxilla are the most common sites of origin. Ameloblastoma with intracranial involvement is a rare presentation with few literature reviews. A long time illness course and multiple surgeries are characteristics present in the majority of cases described. Total resection surgery is the treatment of choice and endoscopic transnasal resection is a viable option.
Keywords: Ameloblastoma, Endoscopy, Skull base surgery, Transsphenoidal
INTRODUCTION
Ameloblastoma is a rare tumor with slow growing and benign behavior. It arises from the dental epithelium and accounts for 1% of all oral tumors.[
Despite benign oncological behavior, ameloblastomas become locally aggressive when the tumor invades the skull base.[
The mandible is the most common site of ameloblastoma. Approximately 75-85% of the cases originate from this bone; the minority originates from maxilla (15-20%).[
The literature shows 14 cases of ameloblastoma with intracranial invasion.[
CASE REPORT
A 19-year-old female patient was referred to our hospital with moderate headache associated with nausea, vomiting, left facial hypoesthesia, and low visual acuity. These symptoms started 2 months before admission. On the neurological exam, Glasgow Coma Scale 15, bilateral papilledema, low visual acuity, left facial hypoesthesia, and absent vomiting reflex were present. Furthermore, after hospitalization, the patient evolved with dysphagia.
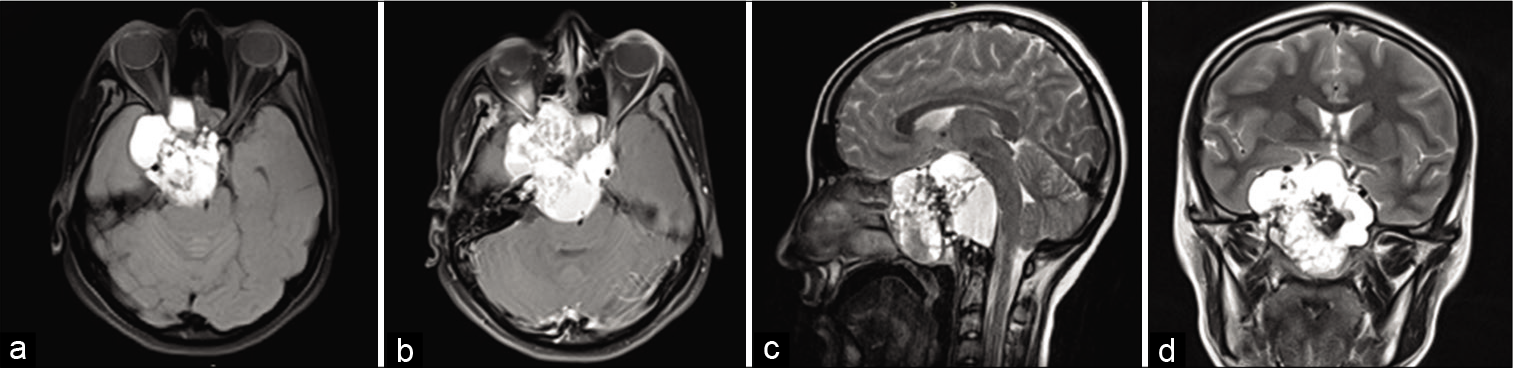
The magnetic resonance image (MRI) [
Figure 1:
Preoperative MRI (a) T1 axial without contrast, (b) T1 axial with contrast, (c) T2 sagittal, (d) T2 coronal – heterogeneous expansive formation involving the skull base, mainly the sphenoid sinus and clivus with neoplastic aspect and dissemination to cavernous sinus, determining compression of the brainstem, surrounding vascular structures, right optical nerve, and optical chiasm.
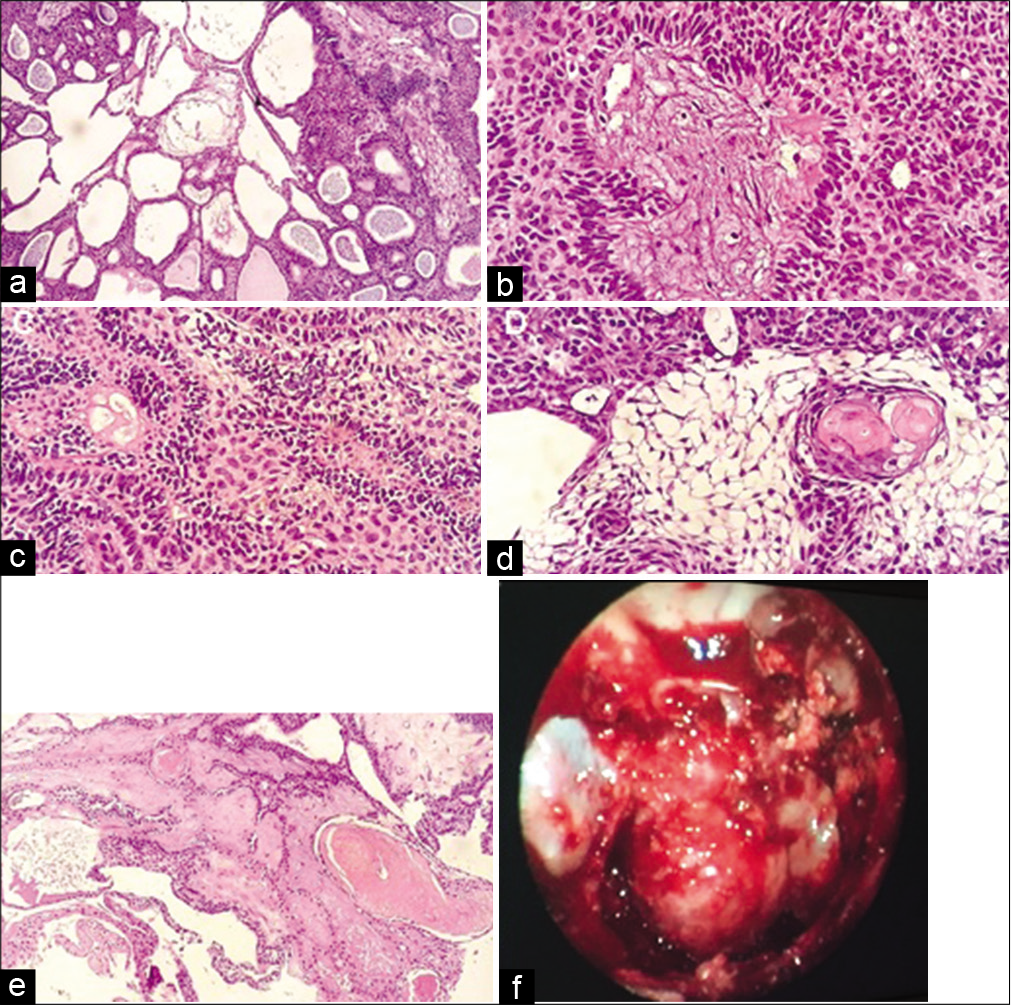
In view of the atypical radiological aspect, we initially opted for an endoscopic transnasal biopsy in August 2017. The pathological study showed odontogenic epithelial islands composed of peripheral palisade columnar cells at basal layer, hyperchromatic. The cells show reverse polarization away from basement membrane (Vickers-Gorlin change). The edematous center mimics the stellate reticulum of the enamel organ. No dentin or enamel formation was found. Other patterns are also seen featuring acanthomatous with squamous metaplasia and variable keratinization of stellate reticulum-like cells, and plexiform with cords and sheets of anastomosing odontogenic epithelial cells. These characteristics defined ameloblastoma as diagnosis [
Figure 2:
The classic histologic features characterized by islands of odontogenic epithelium in fibrous connective tissue; may be cystic (a). Odontogenic epithelial islands composed of peripheral palisading columnar cells at basal layer, hyperchromatic, cells show reverse polarization (b), palisading basal cells and stellate reticulum (c), the central edematous, and mimic the stellate reticulum of the enamel organ (d), featuring acanthomatous with squamous metaplasia (e), heterogeneous lesion with some areas highly calcified (f).
After the biopsy, we concluded that the maximal resection would be the best initial treatment. In September 2017, the patient underwent to a pure endoscopic transnasal transsphenoidal approach to the skull with a total resection of the lesion. There was mild bleeding and the lesion was very heterogeneous with some areas highly calcified [
The initial endoscopic approach was chosen because it allows brainstem and optic nerves decompression, with less risk of damage to nervous and vascular structures, in addition to being a suitable surgical route for resection of the lesion in question when compared with other skull base approaches.
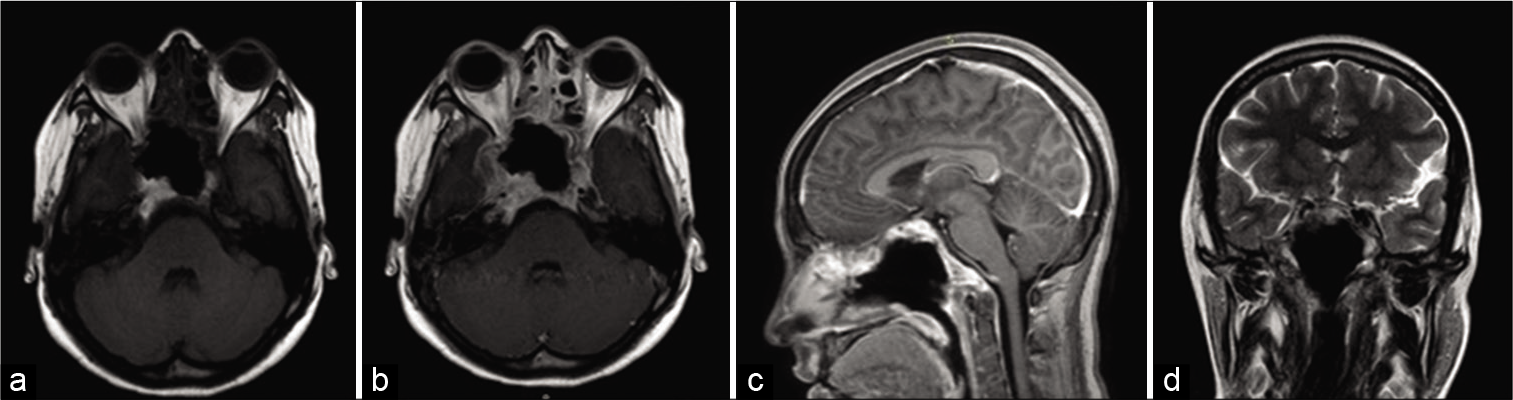
After surgery, the patient presented good evolution and the MRI control image demonstrated excellent local control of tumor [
DISCUSSION
Ameloblastoma is a benign locally invasive lesion responsible for 1% of all oral tumors.[
Epidemiological characteristics are variable in the literature. MAGLIOCA[
The most common sites of origin are mandible and maxilla with 80% and 20%, respectively. The posterior mandible is the most common, responsible for 66% of all cases.[
Usually asymptomatic and with low growth rate, ameloblastomas can be found accidentally in routine dental exams. Rarely presents metastasis, however the skull base, lymph nodes, and the lung are possible metastatic sites. The treatment of these lesions is associated to multiple surgeries and radiation therapy witch is indicated when a subtotal resection of tumor occurs.[
In treatment of ameloblastoma, surgery is the first choice. There is no doubt that the initial extent of ameloblastoma resection is an important factor that influences the rate of recurrence and the prognosis of disease.
In our case, the transnasal endoscopic transsphenoidal approach was successfully used with the inherent benefits to this minimal invasive approach, where no skin incision is required and with reduced manipulation of vascular and nervous tissues, as well satisfactory decompression of the optic nerves and of the brainstem.[
In other cases described in the literature of ameloblastomas with intracranial invasion, the authors used transcranial approaches in treatment, as is described at
Transcranial approaches increase the manipulation of vascular and nervous tissues; however, they allow better control of other structures, in addition to offering a wider route of dissection of the lesion. In our case, the choice was for the endonasal endoscopic approach, because a large resection of tumor, in addition to satisfactory optic nerves and brainstem decompression were possible. Another reason was the bone origin of this tumor. This tumor was centered at sphenoid bone witch facilitated the endoscopic approach.
The radiotherapy is reported as adjuvant therapy in tumors with incomplete surgical resection or with recurrence.[
Low recurrence rates were reported by the authors when a total resection surgery occurs (total tumor resection includes the dental and alveolar structures).[
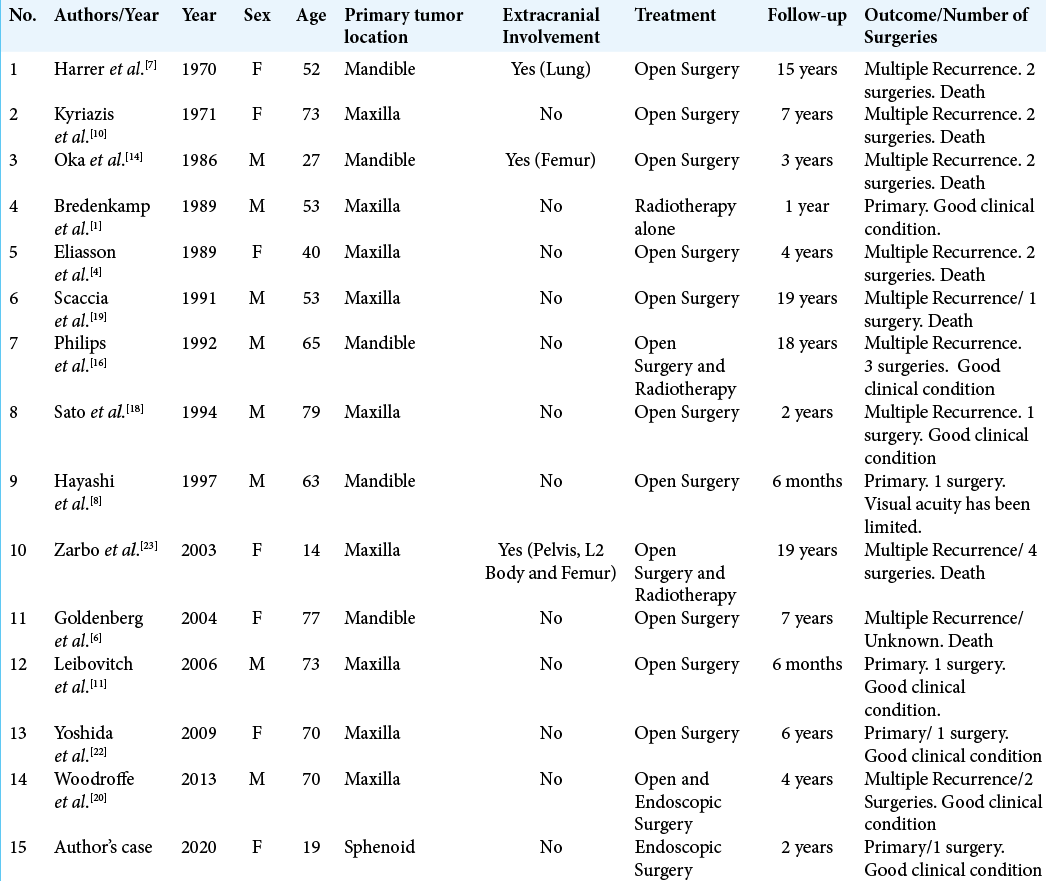
In a literature review, we found 15 cases of ameloblastoma with intracranial invasion including the present case [
CONCLUSION
Ameloblastoma with intracranial involvement is very rare, with only a few cases reported in the literature. Our case is unique due to the presentation in a very young patient of a large ameloblastoma with a probable origin in the sphenoid bone (witch, to the best of our knowledge, has never been reported before under these conditions) presenting an important brainstem distortion that was treated with a pure endoscopic approach.
The surgical approach with total resection of the lesion is the treatment of choice in these cases. Several surgical approaches can be used for treatment aiming at maximum resection. In this article, we present the possibility of a pure endoscopic treatment for ameloblastoma with an intracranial invasion of a patient with more than 2 years of follow-up who still free of the disease.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bredenkamp JK, Zimmerman MC, Mickel RA. Maxillary ameloblastoma. A potentially lethal neoplasm. Arch Otolaryngol Head Neck Surg. 1989. 115: 99-104
2. Brown NA, Rolland D, McHugh JB, Weigelin HC, Zhao L, Lim MS. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014. 20: 5517-26
3. Churchill HR, Ivey RH. The need of a standardized surgical and pathological classification of tumors and anomalies of dental origin. Am Assoc Dent Sch Trans. 1930. 7: 240-5
4. Eliasson AH, Moser RJ, Tenholder MF. Diagnosis and treatment of metastatic ameloblastoma. South Med J. 1989. 82: 1165-8
5. Thawley SE, Panje WR.editors. Comprehensive Management of Head and Neck Tumors. Philadelphia, PA: Saunders Company; 1987. p. 1446-509
6. Goldenberg D, Sciubba J, Koch W, Tufano RP. Malignant odontogenic tumors: A 22-year experience. Laryngoscope. 2004. 114: 1770-4
7. Harrer WV, Patchefsky AS. Mandibular ameloblastoma with intracerebral and pulmonary metastasis. Oral Surg Oral Med Oral Pathol. 1970. 29: 893-8
8. Hayashi N, Iwata J, Masaoka N, Ueno H, Ohtsuki Y, Moriki T. Ameloblastoma of the mandible metastasizing to the orbit with malignant transformation. A histopathological and immunohistochemical study. Virchows Arch. 1997. 430: 501-7
9. Kennedy WR, Werning JW, Kaye FJ, Mendenhall WM. Treatment of ameloblastoma and ameloblastic carcinoma with radiotherapy. Eur Arch Otorhinolaryngol. 2016. 273: 3293-7
10. Kyriazis AP, Karkazis GC, Kyriazis AA. Maxillary ameloblastoma with intracerebral extension. Report of a case. Oral Surg Oral Med Oral Pathol. 1971. 32: 582-7
11. Leibovitch I, Schwarcz RM, Modjtahedi S, Selva D, Goldberg RA. Orbital invasion by recurrent maxillary ameloblastoma. Ophthalmology. 2006. 113: 1227-30
12. Magliocca KR, Steuer CE, Hudgins PA, Bouloux GF. Regarding BRAF-inhibitor therapy of primary ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016. 122: 517-8
13. Malassez L. Sur le rôle des dé-bris épithéliaux paradentaires. Arch Physiol. 1885. 6: 379-449
14. Oka K, Fukui M, Yamashita M, Takeshita I, Fujii K, Kitamura K. Mandibular ameloblastoma with intracranial extension and distant metastasis. Clin Neurol Neurosurg. 1986. 88: 303-9
15. Olaitan AA, Arole G, Adekeye EO. Recurrent ameloblastoma of the jaws. A follow-up study. Int J Oral Maxillofac Surg. 1998. 27: 456-60
16. Phillips SD, Corio RL, Brem H, Mattox D. Ameloblastoma of the mandible with intracranial metastasis. A case study. Arch Otolaryngol Head Neck Surg. 1992. 118: 861-3
17. Quick-Weller J, Koch F, Dinc N, Lescher S, Baumgarten P, Harter P. Intracranial ameloblastoma arising from the maxilla: An interdisciplinary surgical approach. J Neurol Surg A Cent Eur Neurosurg. 2017. 78: 582-7
18. Sato K, Sudo S, Fukuya Y, Sakuma H. Maxillary ameloblastoma with intracranial invasion--case report. Neurol Med Chir. 1994. 34: 704-7
19. Scaccia FJ, Strauss M, Arnold J, Maniglia AJ. Maxillary ameloblastoma: Case report. Am J Otolaryngol. 1991. 12: 20-5
20. Woodroffe RW, Abel TJ, Fletcher A, Grossbach A, Van Daele DJ, O’Brien E. Endoscopic transnasal resection of ameloblastoma with intracranial extension. J Clin Neurosci. 2014. 21: 855-9
21. Yildirim AE, Divanlioglu D, Karaoglu D, Kayacetin S, Belen AD. Massive skull base ameloblastic carcinoma with intracranial extension. Turk Neurosurg. 2017. 27: 1016-20
22. Yoshida K, Kawase T, Tomita T, Ogawa K, Kawana H, Yago K. Surgical strategy for tumors located in or extending from the intracranial space to the infratemporal fossa-advantages of the transcranial approach (zygomatic infratemporal fossa approach) and the indications for a combined transcranial and transcervical approach. Neurol Med Chir (Tokyo). 2009. 49: 580-6
23. Zarbo RJ, Marunick MT, Johns R. Malignant ameloblastoma, spindle cell variant. Arch Pathol Lab Med. 2003. 127: 352-5