- Department of Neurosurgery, Tokyo Medical University, Tokyo, Japan.
Correspondence Address:
Michihiro Kohno, Department of Neurosurgery, Tokyo Medical University, Tokyo, Japan.
DOI:10.25259/SNI_1226_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hiroki Sakamoto, Jiro Akimoto, Masateru Tsutsumi, Ken Matsushima ken, Norio Ichimasu, Michihiro Kohno. Radio-pathological characteristics of malignant transformation of an epidermoid cyst in the cerebellopontine angle: A case report. 08-Apr-2022;13:135
How to cite this URL: Hiroki Sakamoto, Jiro Akimoto, Masateru Tsutsumi, Ken Matsushima ken, Norio Ichimasu, Michihiro Kohno. Radio-pathological characteristics of malignant transformation of an epidermoid cyst in the cerebellopontine angle: A case report. 08-Apr-2022;13:135. Available from: https://surgicalneurologyint.com/surgicalint-articles/11515/
Abstract
Background: Intracranial epidermoid cysts are rare congenital neoplasms that are clinically indolent and histologically benign. They rarely show malignant transformation, and several such cases have been reported. Some radiological features that suggest malignant transformation have been reported. However, histopathological features that indicate a high risk of malignant transformation have not been reported to date.
Case Description: We report a 59-year-old woman with a benign epidermoid cyst in the cerebellopontine angle that showed malignant transformation after 6 years. Magnetic resonance imaging (MRI) at the time of initial onset displayed a high-intensity signal on diffusion-weighted imaging (DWI), no peritumoral edema, and no enhancement on contrast-enhanced T1-weighted imaging. On the other hand, MRI at the time of malignant transformation showed a low-intensity signal on DWI, peritumoral edema, and enhancement of the tumor capsule on contrast-enhanced T1-weighted imaging. Pathological findings at the time of the first surgery differed from normal benign epidermoid cysts, in that stratified squamous epithelial metaplasia was observed, and immunohistochemical (IHC) analysis showed positive p53 staining. In addition, IHC analysis at the time of malignant transformation demonstrated positive p16 staining.
Conclusion: In benign epidermoid cysts, it is considered to cause malignant transformation when squamous metaplasia or p53 mutation is observed. Therefore, strict follow-up is required while paying attention to the characteristic changes in MRI for early detection and timely treatment of malignant transformation.
Keywords: Cerebellopontine angle, Epidermoid cyst, Malignant transformation, p16, p53, Stratified squamous epithelial metaplasia
INTRODUCTION
Intracranial epidermoid cysts are rare congenital neoplasms that are clinically indolent and histologically benign, and account for 0.2–1.8% of all intracranial tumors.[
Here, we report a case of a patient with a cerebellopontine angle (CPA) epidermoid cyst that was determined to be squamous epithelial metaplasia and p53 mutation on histopathology at the first operation. Although the tumor was initially benign, malignant transformation was detected 6 years after the initial operation, with changes on MRI. Characteristics of the cyst that were detected upon the initial immunohistochemical (IHC) analysis were considered to be useful for predicting future malignant transformation. The data of this case before recurrence have already been published in a previous paper.[
CASE REPORT
History and surgical treatment
A 59-year-old woman presented with no relevant past medical history initially presented in 2012 with a 3-year history of right tinnitus, hearing disturbance, and hemifacial paresis. Her right facial function was House–Brackmann (H-B) Grade III, and hearing function was American Academy of Otolaryngology–Head and Neck Surgery (AAOHNS) class B. MRI displayed an extra-axial mass lesion with a maximum diameter of 20 mm in the right CPA. The lesion showed a low-intensity signal on T1-weighted imaging, a high-intensity signal on T2-weighted imaging, and a high-intensity signal on diffusion-weighted imaging (DWI) [
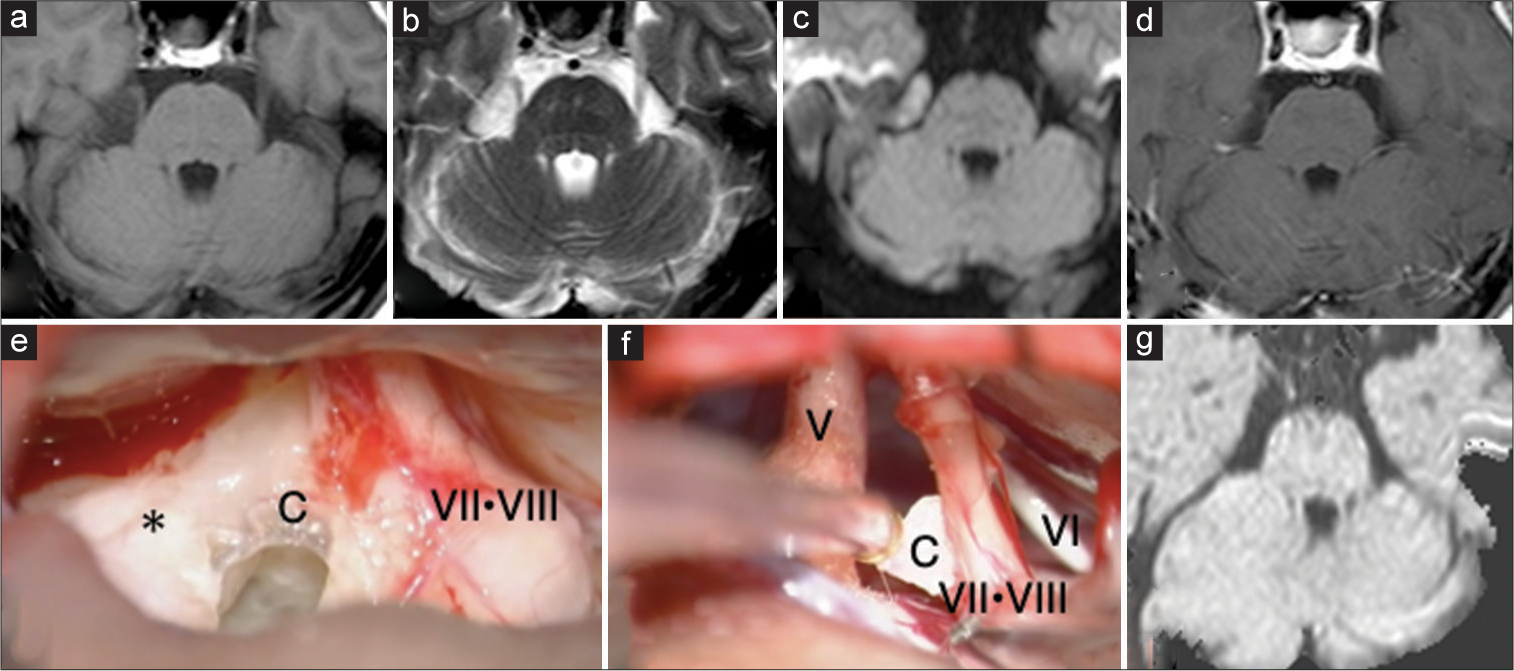
Figure 1:
Magnetic resonance imaging (MRI) and intraoperative findings of a 59-year-old woman with a right cerebellopontine angle epidermoid cyst, before its malignant transformation (a-d) Preoperative MRI displayed a low-intensity signal on T1-weighted imaging, a high-intensity signal on T2-weighted imaging, and a high-intensity signal on diffusion-weighted imaging (DWI) without enhancement on contrast enhanced T1-weighted imaging. (e) The tumor contents (*) were pearly white debris, and the tumor capsule was a translucent thin membrane, most of which could be easily removed from the surrounding tissue. (f) A small portion of the capsule was left because of its tight adhesion to the facial nerve. (g) Postoperative MRI displayed no obvious residual tumor. MRI sequences in (a) is T1-weighted images. MRI sequences in (b) are T2-weighted images. MRI sequences in (c and g) are DWI. MRI sequences in (d) are contrast-enhanced T1-weighted images. C=Tumor capsule; V=Trigeminal nerve; VI=Abducens nerve; VII=Facial nerve; VIII=Vestibulocochlear nerve.
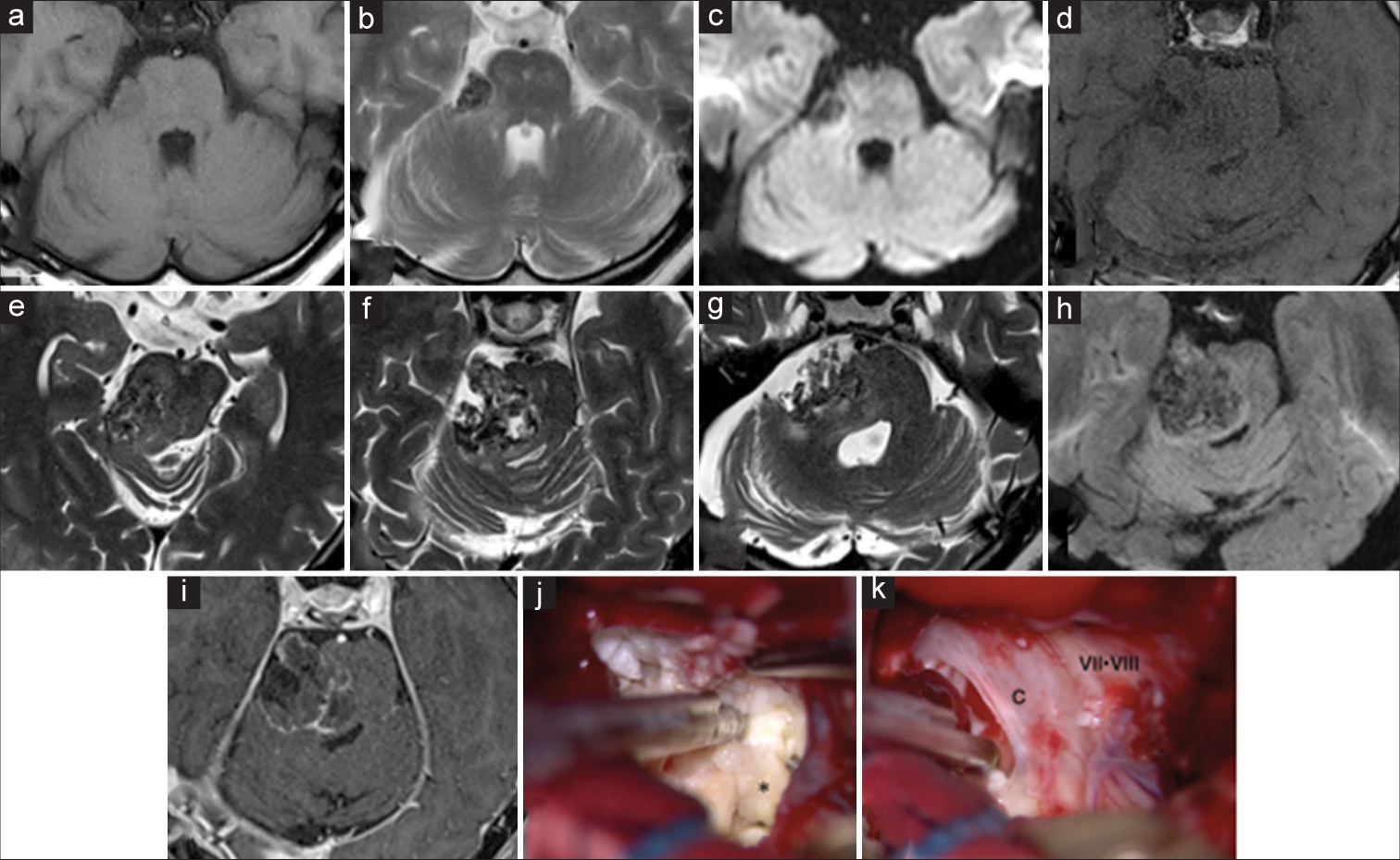
Figure 2:
Magnetic resonance imaging (MRI) and intraoperative findings of a 59-year-old woman with a right cerebellopontine angle epidermoid cyst, after its malignant transformation (a-c) MRI 6 years after the initial surgery displayed slight characteristics of recurrence. The lesion showed a low-intensity signal on T1-weighted imaging, a heterogeneous low-intensity signal on T2-weighted imaging, and a low-intensity signal on DWI. (d-i) MRI 8 years after the initial surgery displayed a contrast effect on the tumor capsule, suggesting malignant transformation. (j) The tumor contents (*) were yellowish and flaky debris, unlike that of the initial surgery. (k) The tumor capsule was a thick and cloudy membrane that tightly adhered to the surrounding cranial nerves and brainstem. MRI sequences in (a and d) are T1-weighted images. MRI sequences in (b and e-g) are T2-weighted images. MRI sequences in (c) and (h) are DWI. MRI sequences in (i) is contrast-enhanced T1-weighted images. C=Tumor capsule; VII=Facial nerve; VIII=Vestibulocochlear nerve.
Histopathology
At the time of the first surgery, histological analysis revealed that the cyst lining was composed of stratified squamous epithelium with a granular layer and abundant lamellated keratin flakes. The histological diagnosis was an epidermoid cyst. The cyst lining demonstrated multilayered squamous metaplasia owing to the proliferation of basal cells with mild nuclear atypia [
At the time of the second surgery, histological analysis revealed that the cyst lining had typical epidermoid features, and in many parts the cyst lining showed multilayered squamous metaplasia with atypical features, such as the loss of cellular polarity and few mitotic figures [
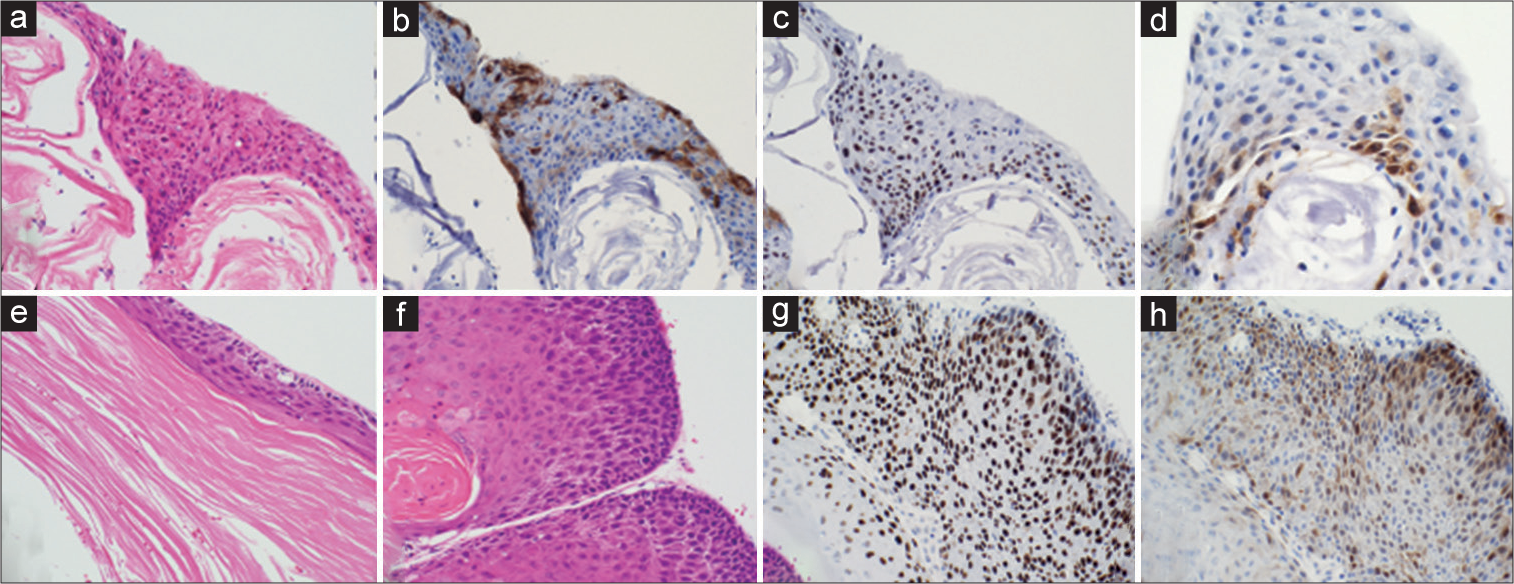
Figure 3:
Histopathological findings of the tumors removed at the first (a-d) and second (e-h) surgeries (a) Hematoxylin and eosin staining showing a cyst with a lining composed of stratified squamous epithelium with a granular layer and abundant lamellated keratin flakes. The squamous epithelium shows multilayered squamous metaplasia in some regions, resulting from the proliferation of basal cells. These squamous cells show mild atypical features and loss of cell polarity (original magnification: ×200). (b-d) Immunohistochemical analysis (b: Epithelial membrane antigen (EMA), ×200; c: p53, ×200; d: p16, ×400). The stratified squamous epithelial cells show partial positive staining for EMA, and 70% to 80% of the cells were positive for p53 and 10% to 20% of the cells were positive for p16. (e and f) Hematoxylin and eosin staining showing the cyst lining composed of stratified squamous epithelium with a granular layer and abundant lamellated keratin flakes. (e) In many parts of the cyst lining, the squamous epithelium shows multilayered metaplasia of atypical squamous cells, with a few mitotic figures (e and f: original magnification ×200) (g and h) Immunohistochemical analysis (g: p53, ×200; h: p16, ×200). Almost all atypical squamous cells were positive for p53, and 30–40% of the cells were positive for p16.
DISCUSSION
Intracranial epidermoid cysts are congenital lesions caused by ectodermal cell migration during neural tube closure, at 3–5 weeks of gestation.[
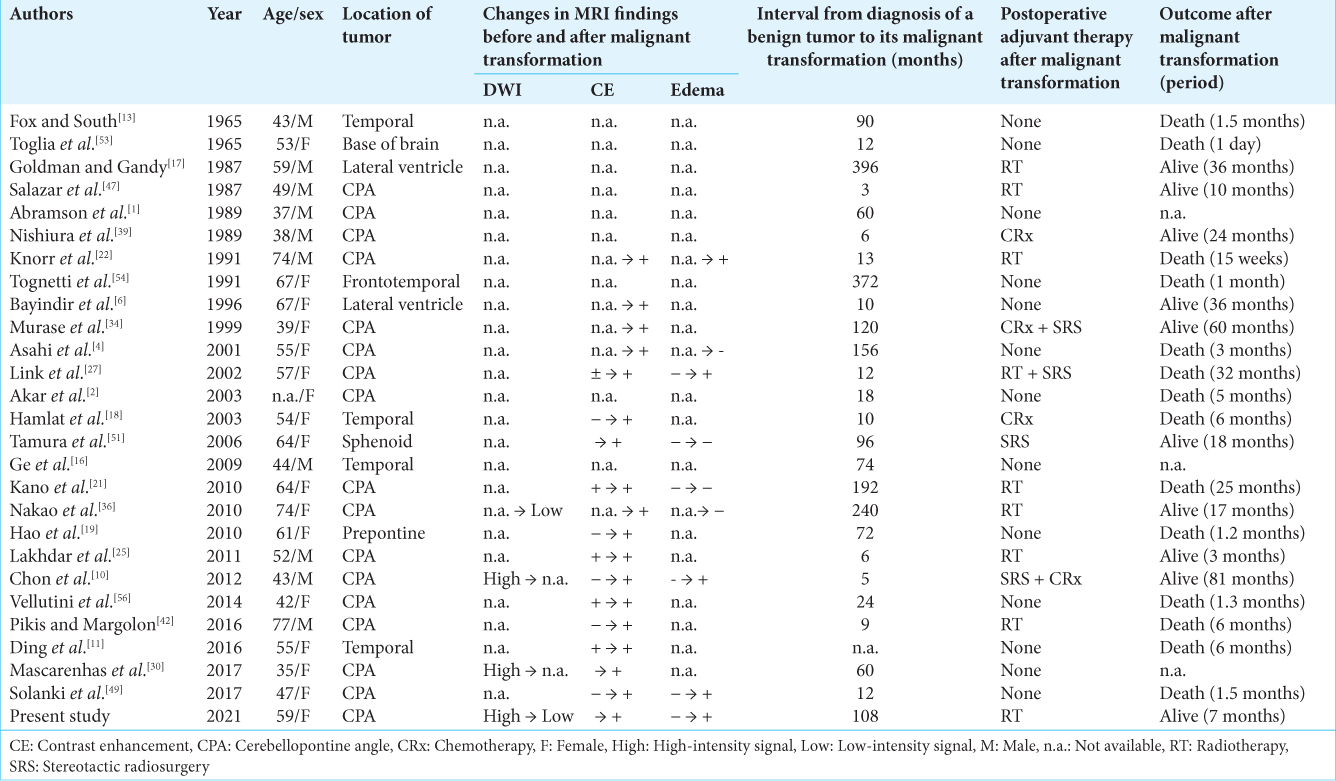
Imaging findings that are suggestive of the malignant transformation of a benign intracranial epidermoid cyst is the appearance of rapid growth, peritumoral edema, tissue invasion, low-intensity signal on DWI, and the appearance of new enhancement following contrast administration[
At the time of reoperation, the tumor capsule was tightly adhered to the surrounding tissue, so the removal rate was lower than that at the first surgery. However, this finding is not specific to malignant transformation because the tumor capsule becomes more difficult to remove also during the reoperation of benign epidermoid cysts.[
Regarding postoperative adjuvant therapy for the malignant epidermoid cysts, as a review by Kwon et al.[
The difference from the pathological findings of a typical benign epidermoid cyst at the time of initial surgery in our patient was that multilayered squamous metaplasia caused by the proliferation of basal cells with mild nuclear atypia, and loss of cellular polarity without mitotic figures were observed. In a previous report, Bayindir et al.[
CONCLUSION
Although epidermoid cysts are benign, it is considered to cause malignant transformation when squamous metaplasia or p53 mutation is observed. Therefore, in such cases, strict follow-up is required while paying attention to the characteristic changes in MRI for early detection and timely treatment of malignant transformation.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are indebted to Helena Akiko Popiel, Department of International Medical Communications of Tokyo Medical University for her review of the English manuscript. And we thank Ms. Eriko Hikawa for her assistance in preparing this manuscript.
References
1. Abramson RC, Morawetz RB, Schlitt M. Multiple complications from an intracranial epidermoid cyst: Case report and literature review. Neurosurgery. 1989. 24: 574-8
2. Akar Z, Tanriover N, Tuzgen S, Kafadar AM, Kuday C. Surgical treatment of intracranial epidermoid tumors. Neurol Med Chir (Tokyo). 2003. 43: 275-81
3. Annet L, Duprez T, Grandin C, Dooms G, Collard A, Cosnard G. Apparent diffusion coefficient measurements within intracranial epidermoid cysts in six patients. Neuroradiology. 2002. 44: 326-8
4. Asahi T, Kurimoto M, Endo S, Monma F, Ohi M, Takami M. Malignant transformation of cerebello-pontine angle epidermoid. J Clin Neurosci. 2001. 8: 572-4
5. Baumann CH, Bucy PC. Paratrigeminal epidermoid tumors. J Neurosurg. 1956. 13: 455-68
6. Bayindir C, Balak N, Karasu A. Micro-invasive squamous cell carcinoma arising in a pre-existing intraventricular epidermoid cyst. Acta Neurochir (Wien). 1996. 138: 1008-12
7. Berger MS, Wilson CB. Epidermoid cysts of the posterior fossa. J Neurosurg. 1985. 62: 214-9
8. Blahak J, Zelinka J, Gumulec J, Machacek C, Danek Z, Bulik O. HPV, protein p16 and squamous cell carcinoma of the oral cavity. Biomed Pap. 2020. 164: 292-9
9. Chandler WF, Farhat SM, Pauli FJ. Intrathalamic epidermoid tumor. J Neurosurg. 1975. 43: 614-7
10. Chon KH, Lee JM, Koh EJ, Choi HY. Malignant transformation of an epidermoid cyst in the cerebellopontine angle. J Korean Neurosurg Soc. 2012. 52: 148-51
11. Ding S, Jin Y, Jiang J. Malignant transformation of an epidermoid cyst in the temporal and prepontine region: Report of a case and differential diagnosis. Oncol Lett. 2016. 11: 3097-100
12. Ernst-Heidelberg P. Accumulation of dysontogenetic formations in the central nervous system. Verh Dtsch Pathlo Ges. 1912. 15: 1226-30
13. Fox H, South EA. Squamous cell carcinoma developing in an Intracranial epidermoid cyst (cholesteatoma). J Neurol Neurosurg Psychiatry. 1965. 28: 276-81
14. Freed-Pastor WA, Prives C. Mutant p53: One name, many proteins. Genes Dev. 2012. 26: 1268-86
15. Gao P, Osborn AG, Smirniotopoulos JG, Harris CP. Radiologic-pathologic correlation. Epidermoid tumor of the cerebellopontine angle. Am J Neuroradiol. 1992. 13: 863-72
16. Ge P, Luo Y, Fu S, Ling F. Recurrent epidermoid cyst with malignant transformation into squamous cell carcinoma case report. Neurol Med Chir (Tokyo). 2009. 49: 442-4
17. Goldman SA, Gandy SE. Squamous cell carcinoma as a late complication of intracerebroventricular epidermoid cyst. Case report. J Neurosurg. 1987. 66: 618-20
18. Hamlat A, Hua ZF, Saikali S, Egreteau J, Guegan Y. Malignant transformation of intracranial epidermoid cyst with leptomeningeal carcinomatosis: Case report. Acta Neurol Belg. 2003. 103: 221-4
19. Hao S, Tang J, Wu Z, Zhang L, Zhang J, Wang Z. Natural malignant transformation of an intracranial epidermoid cyst. J Formos Med Assoc. 2010. 109: 390-6
20. Inoue K, Fry EA. Aberrant expression of p16 INK4a in human cancers a new biomarker?. Cancer Rep Rev. 2018. 2: 145
21. Kano T, Ikota H, Kobayashi S, Iwasa S, Kurosaki S, Wada H. Malignant transformation of an intracranial large epidermoid cyst with leptomeningeal carcinomatosis. Neurol Med Chir (Tokyo). 2010. 50: 349-53
22. Knorr JR, Ragland RL, Smith TW, Davidson RI, Keller JD. Squamous carcinoma arising in a cerebellopontine angle epidermoid: CT and MR findings. Am J Neuroradiol. 1991. 12: 1182-4
23. Kodama H, Maeda M, Hirokawa Y, Suzuki H, Hori K, Taki W. MRI findings of malignant transformation of epidermoid cyst: Case report. J Neurooncol. 2007. 82: 171-4
24. Kwon SM, Kim JH, Kim YH, Hong SH, Kim CJ. Treatment and survival outcomes of primary intracranial squamous cell carcinoma. World Neurosurg. 2019. 125: E1-9
25. Lakhdar F, Hakkou EM, Gana R, Maaqili RM, Bellakhdar F. Malignant transformation six months after removal of intracranial epidermoid cyst: A case report. Case Rep Neurol Med. 2011. 2011: 525289
26. Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979. 278: 261-3
27. Link MJ, Cohen PL, Breneman JC, Tew JM. Malignant squamous degeneration of a cerebellopontine angle epidermoid tumor. J Neurosurg. 2002. 97: 1237-43
28. Linzer DIH, Levine AJ. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979. 17: 43-52
29. Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010. 11: 781-9
30. Mascarenhas A, Parsons A, Smith C, Molloy C, Jukes A. Malignant squamous cell carcinoma arising in a previously resected cerebellopontine angle epidermoid. Surg Neurol Int. 2017. 8: 186
31. Meszaros N, Belengeanu D, Stoicănescu D, Andreescu N, Farcaş S, Stoian M. Analyses of numerical aberrations of chromosome 17 and tp53 gene deletion/amplification in human oral squamous cell carcinoma using dual-color fluorescence in situ hybridization. Tom. 2010. 17: 142-6
32. Moran CC, Vakili ST, Caldemeyer KS, Smith RR. Foreign body giant cell reaction associated with epidermoid tumor: CT and MR findings. J Comput Assist Tomogr. 1995. 19: 628-30
33. Munger K, Gwin TK, McLaughlin-Drubin M. p16 in HPV-associated cancers. Oncotarget. 2013. 4: 1864-5
34. Murase S, Yamakawa H, Ohkuma A, Sumi Y, Kajiwara M, Takami T. Primary intracranial squamous cell carcinoma-case report. Neurol Med Chir (Tokyo). 1999. 39: 49-54
35. Nagasawa D, Yew A, Safaee M, Fong B, Gopen Q, Parsa AT. Clinical characteristics and diagnostic imaging of epidermoid tumors. J Clin Neurosci. 2011. 18: 1158-62
36. Nakao Y, Nonaka S, Yamamoto T, Oyama K, Esaki T, Tange Y. Malignant transformation 20 years after partial removal of intracranial epidermoid cyst-case report. Neurol Med Chir (Tokyo). 2010. 50: 236-9
37. Nawashiro H, Higo R, Tokumaru AM, Tsuzuki N, Shima K. Diffusion-weighted MRI of an intracranial epidermoid with malignant transformation. Neuroradiology. 2001. 43: 891
38. Nishio S, Takeshita I, Morioka T, Fukui M. Primary intracranial squamous cell carcinomas: Report of two cases. Neurosurgery. 1995. 37: 329-32
39. Nishiura I, Koyama T, Handa J, Amano S. Primary intracranial epidermoid carcinoma-case report. Neurol Med Chir (Tokyo). 1989. 29: 600-5
40. Obrador S, Lopez-Zafra JJ. Clinical features of the epidermoids of the basal cisterns of the brain. J Neurol Neurosurg Psychiatry. 1969. 32: 450-4
41. Ozutemiz C, Ada E, Ersen A, Ozer E. Imaging findings of an epidermoid cyst with malignant transformation to squamous cell carcinoma. Turk Neurosurg. 2017. 27: 312-5
42. Pikis S, Margolin E. Malignant transformation of a residual cerebellopontine angle epidermoid cyst. J Clin Neurosci. 2016. 33: 59-62
43. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018. 78: 237-47
44. Reis GF, Pekmezci M, Hansen HM, Rice T, Marshall RE, Molinaro AM. CDKN2A loss is associated with shortened overall survival in lower grade (World Health Organization IIIII) astrocytomas. J Neuropathol Exp Neurol. 2015. 74: 442-52
45. Rothschild S, Ciernik IF, Hartmann M, Schuknecht B, Lütolf UM, Huber AM. Cholesteatoma triggering squamous cell carcinoma: case report and literature review of a rare tumor. Am J Otolaryngol Head Neck Med Surg. 2009. 30: 256-60
46. Sakamoto H, Kohno M, Matsushima K, Ichimasu N, Nakajima N, Yoshino M. Importance of appropriate surgical approach selection for radical resection of cerebellopontine angle epidermoid cysts with preservation of cranial nerve functions: our experience of 54 cases. Acta Neurochir (Wien). 2021. 163: 2465-74
47. Salazar J, Vaquero J, Saucedo G, Bravo G. Posterior fossa epidermoid cysts. Acta Neurochir. 1987. 85: 34-9
48. Shear BM, Jin L, Zhang Y, David WB, Fomchenko EI, ErsonOmay EZ. Extent of resection of epidermoid tumors and risk of recurrence: case report and meta-analysis. J Neurosurg. 2019. 5: 1-11
49. Solanki SP, Maccormac O, Dow GR, Smith S. Malignant transformation of residual posterior fossa epidermoid cyst to squamous cell carcinoma. Br J Neurosurg. 2017. 31: 497-8
50. Soufir N, Queille S, Liboutet M, Thibaudeau O, Bachelier F, Delestaing G. Inactivation of the CDKN2A and the p53 tumour suppressor genes in external genital carcinomas and their precursors. Br J Dermatol. 2007. 156: 448-53
51. Tamura K, Aoyagi M, Wakimoto H, Tamaki M, Yamamoto K, Yamamoto M. Malignant transformation eight years after removal of a benign epidermoid cyst: A case report. J Neurooncol. 2006. 79: 67-72
52. Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005. 86: 347-63
53. Toglia JU, Netsky MG, Alexander E. Epithelial (epidermoid) tumors of the cranium. Their common nature and pathogenesis. J Neurosurg. 1965. 23: 384-93
54. Tognetti F, Lanzino G, Manetto V, Calbucci F. Intracranial squamous cell carcinoma arising in remnant of extirpated epidermoid cyst. Br J Neurosurg. 1991. 5: 303-5
55. Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: An autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019. 15: 1429-39
56. Vellutini EA, De Oliveira MF, Ribeiro AP, Rotta JM. Malignant transformation of intracranial epidermoid cyst. Br J Neurosurg. 2014. 28: 507-9
57. Zedan W, Mourad MI, El-Aziz SM, Salamaa NM, Shalaby AA. Cytogenetic significance of chromosome 17 aberrations and P53 gene mutations as prognostic markers in oral squamous cell carcinoma. Diagn Pathol. 2015. 10: 2
58. Zur Hausen H. Papillomaviruses causing cancer: Evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000. 92: 690-8