- Department of Neurosurgery, Tokyo Women’s Medical University, Tokyo, Japan.
Correspondence Address:
Shiro Horisawa, Department of Neurosurgery, Tokyo Women’s Medical University, Tokyo, Japan.
DOI:10.25259/SNI_311_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Shiro Horisawa, Kilsoo Kim, Makiko Sakaguchi, Takakazu Kawamata, Takaomi Taira. Radiofrequency ablation of the pallidothalamic tract and ventral intermediate nucleus for dystonic tremor through the parietal approach. 03-Nov-2023;14:390
How to cite this URL: Shiro Horisawa, Kilsoo Kim, Makiko Sakaguchi, Takakazu Kawamata, Takaomi Taira. Radiofrequency ablation of the pallidothalamic tract and ventral intermediate nucleus for dystonic tremor through the parietal approach. 03-Nov-2023;14:390. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12621
Abstract
Background: The thalamic ventral intermediate nucleus (Vim) and globus pallidus internus are far apart and cannot be captured using a single electrode.
Case Description: We describe our experience with a patient with dystonic tremors of the head and upper and lower extremities who showed symptomatic improvement after radiofrequency (RF) ablation using a parietal lobe approach with a single trajectory to capture the pallidothalamic tract and Vim. A 46-year-old man developed head tremors at 41 and a right-sided neck tilt three years later. Five years after the onset of the head tremors, tightness of the larynx during speech and tremors in both the upper and lower limbs also appeared. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) score was 24, and the Fahn-Tolosa-Marin Tremor Rating Scale (FTM) score was 48. We captured the pallidothalamic tract and Vim along a single trajectory by locating the entry point in the inferior parietal lobule. One week after treatment, the TWSTRS and FTM scale scores were 9 (62.5%) and 30 (37.5%), respectively. No adverse events were observed.
Conclusion: This case suggests that in dystonic tremors involving abnormalities of the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits, a single electrode can be used to approach both circuits through the parietal lobe approach.
Keywords: Dystonic tremor, Pallidothalamic tract, Parietal lobe, Ventral intermediate nucleus
INTRODUCTION
The optimal target for the stereotactic neurosurgical treatment of dystonic tremors has not yet been established. Deep brain stimulation (DBS) of the ventral intermediate nucleus (Vim) is the preferred surgical treatment for dystonic tremors.[
CASE REPORT
A 46-year-old man developed head tremors at 41 and a right-sided neck tilt three years later. Five years after the onset of the head tremors, tightness of the larynx during speech and tremors in both the upper and lower limbs also appeared. Because the symptoms were refractory to oral medications, the patient was referred to our department for surgical treatment. He had no history of head trauma, treatment for psychiatric disorders, or a family history of movement disorders. Magnetic resonance imaging (MRI) of the head revealed no apparent structural abnormalities. The patient’s symptoms included right lateral flexion and forward neck bending, horizontal head tremors, and tremors in both the upper and lower extremities [
Video 1
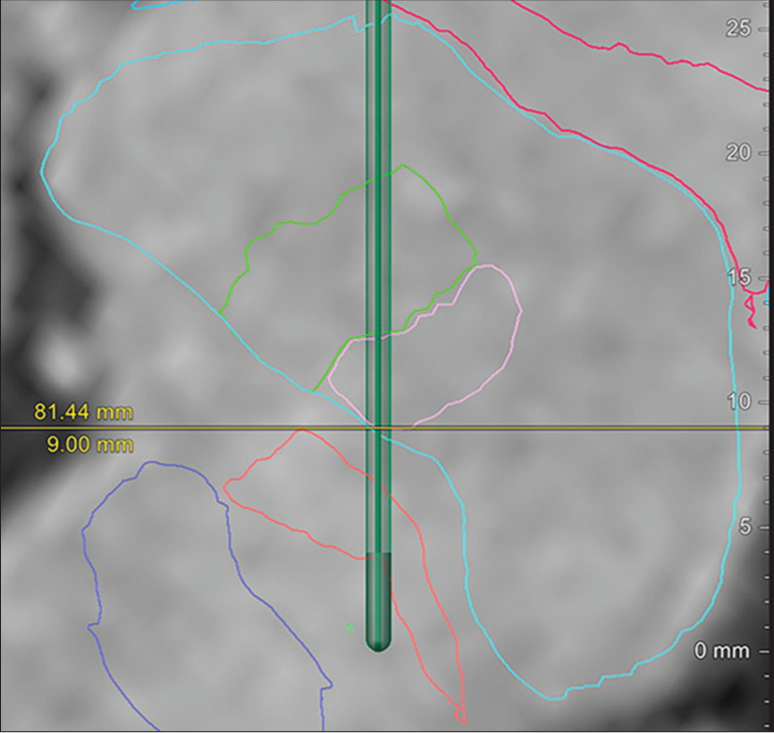
BrainLab elements were used for the surgical planning. The targets were the left PTT for cervical dystonia due to the right-sided neck flexion and the left Vim nucleus for the right upper extremity tremor. The electrode tip for the left PTT was set at 8 mm lateral, 0.5 mm posterior, and 3 mm inferior to the midpoint of the anterior commissure-posterior commissure. We captured the PTT and Vim along a single trajectory by locating the entry point in the inferior parietal lobule [
Figure 2:
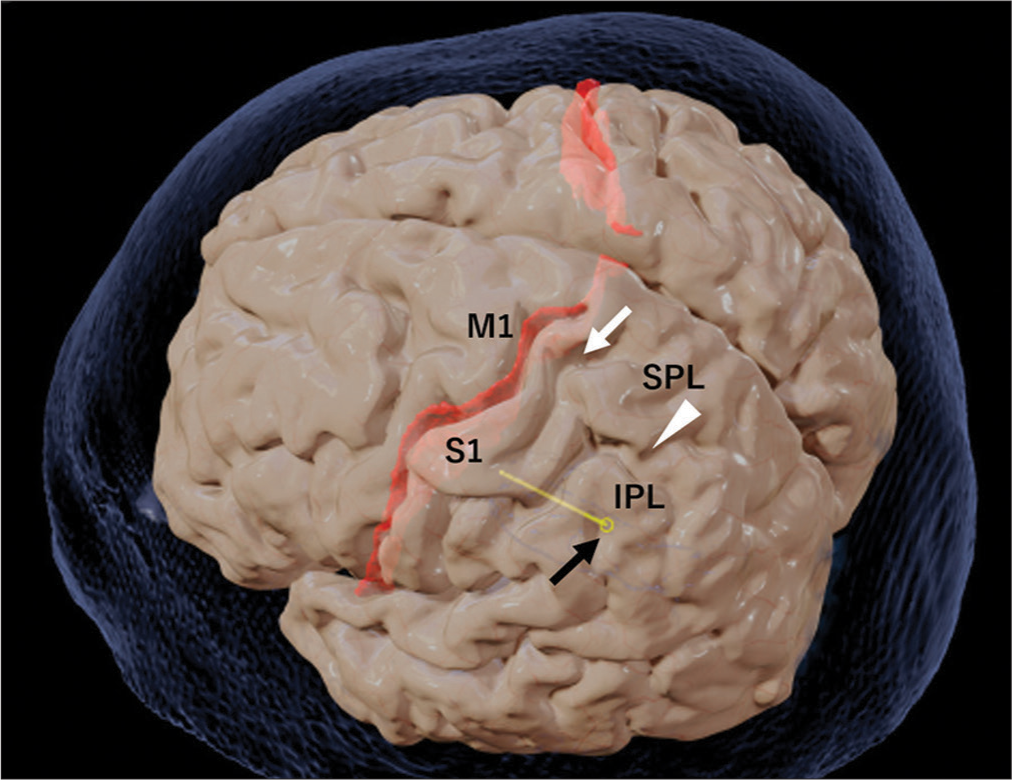
Entry point of the electrode on the brain surface. Black arrow: Electrode (yellow) entry point on the brain surface. White arrow: Post-central sulcus. White arrowhead: Intra-parietal sulcus. The electrode entry point is at the inferior parietal lobule (IPL). M1: Primary motor cortex; S1: Primary somatosensory cortex; SPL: Superior parietal lobule.
Video 2
Postoperative MRI revealed lesions in the PTT and Vim nuclei, as per the preoperative surgical plan [
Figure 3:
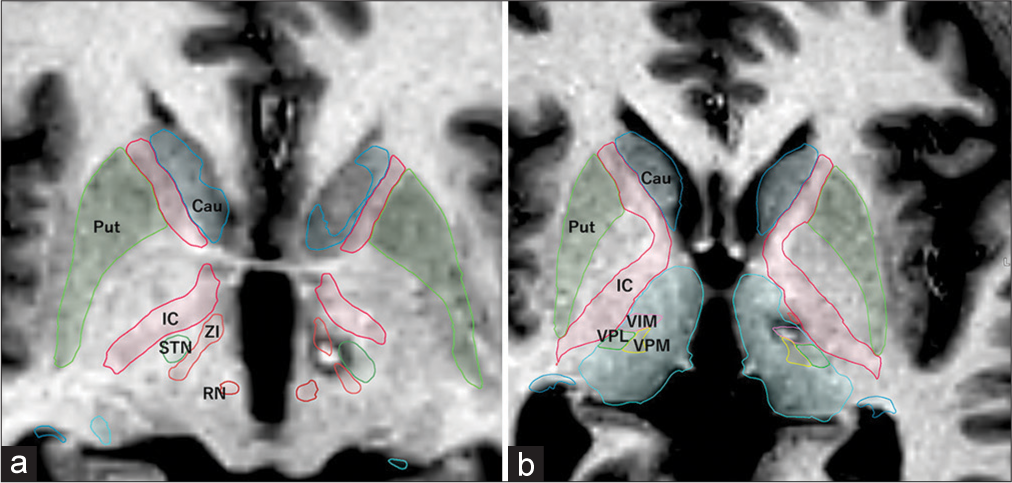
Postoperative T1-weighted MRI at the PTT and Vim nucleus level. (a) Lesion of the left PTT located medial to the STN and anterior to the RN. (b) Lesion of the left Vim nucleus located within the Vim nucleus, as simulated by anatomical mapping of the BrainLab elements. PTT: Pallidothalamic tract, MRI: Magnetic resonance imaging, Cau: Caudate nucleus, Put: putamen, IC: Internal capsule, RN: Red nucleus, STN: Subthalamic nucleus, Vim: Ventral intermediate, VPL: Ventroposterolateral nucleus, VPM: Ventroposteromedial nucleus, ZI: Zona incerta.
Video 3
DISCUSSION
We applied the parietal approach to capture the PTT and Vim in a single trajectory, and RF thermocoagulation of the PTT and Vim improved cervical dystonia and tremor in the right upper extremity in this case. Using a single trajectory for the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits is a meaningful approach for treating dystonic tremors.
Studies examining functional connectivity using resting-state functional MRI reported a wider range of functional connectivity abnormalities for dystonic tremors compared to essential tremors.[
Attempts to capture two different circuits with a single electrode have been reported. Buhmann et al. performed DBS in three patients with dystonic head tremors.[
We previously reported the application of Vo-Vim DBS for head tremors by implanting a DBS with a steep angle, in which head tremors disappeared with stimulation of the VopVim border.[
Coenen et al. conducted an earlier conceptual study of the parietal approach.[
Multiple target lesions have been reported for the treatment of dystonia. Hassler et al. performed unilateral Forel H1, Voi, and Voa lesioning for cervical dystonia.[
CONCLUSION
The present case suggests that in dystonic tremors involving abnormatilites of the basal ganglia-thalamo-cortical and cerebello-thalamo-cortical circuits, a single electrode can be used to approach both circuits through the parietal lobe approach.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Videos available on:
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Buhmann C, Moll CK, Zittel S, Münchau A, Engel AK, Hamel W. Deep brain stimulation of the ventrolateral thalamic base and posterior subthalamic area in dystonic head tremor. Acta Neurochir Suppl. 2013. 117: 67-72
2. Coenen VA, Rijntjes M, Prokop T, Piroth T, Amtage F, Urbach H. One-pass deep brain stimulation of dentato-rubro-thalamic tract and subthalamic nucleus for tremor-dominant or equivalent type Parkinson’s disease. Acta Neurochir (Wien). 2016. 158: 773-81
3. DeSimone JC, Archer DB, Vaillancourt DE, Wagle Shukla A. Network-level connectivity is a critical feature distinguishing dystonic tremor and essential tremor. Brain. 2019. 142: 1644-59
4. Hassler R, Dieckmann G. Stereotactic treatment of different kinds of spasmodic torticollis. Confin Neurol. 1970. 32: 135-43
5. Hassler R, editors. Stereotaxic treatment for spasmodic torticollis. Stereotaxy of the human brain. Stuttgart New York: G Thieme; 1982. p. 522-31
6. Horisawa S, Fukui A, Kohara K, Kawamata T, Taira T. Unilateral pallidotomy in the treatment of cervical dystonia: A retrospective observational study. J Neurosurg. 2019. 134: 216-22
7. Horisawa S, Fukui A, Takeda N, Kawamata T, Taira T. Safety and efficacy of unilateral and bilateral pallidotomy for primary dystonia. Ann Clin Transl Neurol. 2021. 8: 857-65
8. Horisawa S, Kohara K, Nonaka T, Fukui A, Mochizuki T, Iijima M. Unilateral pallidothalamic tractotomy at Forel’s field H1 for cervical dystonia. Ann Clin Transl Neurol. 2022. 9: 478-87
9. Loher TJ, Pohle T, Krauss JK. Functional stereotactic surgery for treatment of cervical dystonia: Review of the experience from the lesional era. Stereotact Funct Neurosurg. 2004. 82: 1-13
10. Paoli D, Mills R, Brechany U, Pavese N, Nicholson C. DBS in tremor with dystonia: VIM, GPi or both? A review of the literature and considerations from a single-center experience. J Neurol. 2023. 270: 2217-29
11. Savas A, Bayatli E, Eroglu U, Akbostanci MC. Combined unilateral radiofrequency lesioning of the motor thalamus, field of Forel, and zona incerta: A series of cases with dystonia. Neurosurgery. 2022. 90: 313-21
12. Trompette C, Giordana C, Leplus A, Grabli D, Hubsch C, Marsé C. Combined thalamic and pallidal deep brain stimulation for dystonic tremor. Parkinsonism Relat Disord. 2022. 103: 29-33
13. Tsuboi T, Au KL, Deeb W, Almeida L, Foote KD, Okun MS. Motor outcomes and adverse effects of deep brain stimulation for dystonic tremor: A systematic review. Parkinsonism Relat Disord. 2020. 76: 32-41
14. Tsuboi T, Wong JK, Eisinger RS, Okromelidze L, Burns MR, Ramirez-Zamora A. Comparative connectivity correlates of dystonic and essential tremor deep brain stimulation. Brain. 2021. 144: 1774-86
15. Yamahata H, Horisawa S, Hodotsuka K, Kawamata T, Taira T. Long-term successful outcome of dystonic head tremor after bilateral deep brain stimulation of the ventral intermediate and ventro-oral internus nuclei: A case report and literature review of dystonic head tremor. Stereotact Funct Neurosurg. 2021. 99: 107-12