- Department of Neurosurgery, University of Caxias do Sul, Caxias do Sul, RS, Brazil
- Medical Faculty of University of Caxias do Sul, University of Caxias do Sul, Caxias do Sul, RS, Brazil
- Cell Therapy Laboratory, University of Caxias do Sul, Caxias do Sul, RS, Brazil
- Clinical Studies and Basic Models of Spinal Disorders Laboratory, University of Caxias do Sul, Caxias do Sul, RS, Brazil
- Department of Neurosurgical, AOSpine, Latin America
- Department of Neurosurgical, Institute of Cancer of the State of São Paulo, São Paulo, SP, Brazil
Correspondence Address:
Asdrubal Falavigna
Department of Neurosurgical, Institute of Cancer of the State of São Paulo, São Paulo, SP, Brazil
DOI:10.4103/2152-7806.176131
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Falavigna A, da Silva PG, Teixeira W. Radiotherapy-induced tumors of the spine, peripheral nerve, and spinal cord: Case report and literature review. Surg Neurol Int 10-Feb-2016;7:
How to cite this URL: Falavigna A, da Silva PG, Teixeira W. Radiotherapy-induced tumors of the spine, peripheral nerve, and spinal cord: Case report and literature review. Surg Neurol Int 10-Feb-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/radiotherapy%e2%80%91induced-tumors-of-the-spine-peripheral-nerve-and-spinal-cord-case-report-and-literature-review/
Abstract
Background:The development of a secondary malignancy in the field of radiation is a rare but well-recognized hazard of cancer treatment. The radiotherapy-induced (RT-I) tumors are even more aggressive and potentially lethal than the primary tumor. To goal of this article is to report a case of RT-I neural tumor located in the peripheral nerve and spinal cord and to perform a literature review of the subject.
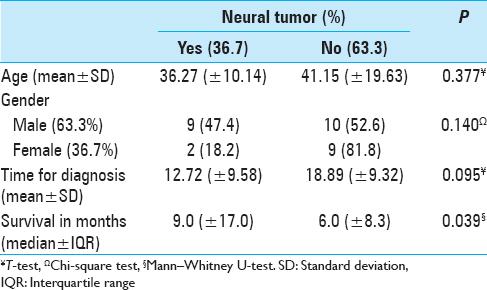
Case Reports:Thirty-year male with symptoms of hypoesthesia and dysesthesia of the L5 nerve root distribution and previous treatment of a testicular seminoma 20 years previously. The lumbar magnetic resonance imaging showed the growth of a nerve root tumor. Surgery was performed, and a fusiform tumor was resected with clear margins. The anatomopathological and immunohistochemical studies were compatible with a malignant peripheral nerve sheath tumor. A total of 30 cases were included in the review. The mean age of the patients at diagnosis of the induced tumor was 39.36 (±16.74) years. Most were male (63.3%). The main type of primary disease was neural tumors (30%). The most common type of histology was fibrosarcoma (20.0%). No difference was found in age, gender, and time of diagnosis between neural and nonneural tumors. The mean survival after the diagnosis of the secondary tumor was 10.7 months (±13.27), and neural tumors had a longer survival period (P = 0.031).
Conclusion:The current gold standard therapy is complete resection with clear margins, since most tumors do not respond to chemotherapy and RT. The neural type of RT-I tumor presented a longer survival period.
Keywords: Malignant peripheral nerve sheath tumor, radiation-induced tumor, radiotherapy, spine, spine surgery
INTRODUCTION
The development of a secondary malignancy in the field of radiation is a rare but well-recognized hazard of cancer treatment.[
Even though the risk for developing a new tumor is low, varying from 0.9% to 2%,[
Usually, the site of the RT-I tumor is the periphery of the radiation field.[
RT-I tumors have become increasingly important because of the longer life of the general population, even the oncologic patients. The goal of this study is to report a case of neural tumor secondary to RT and to perform a literature review on the cases of RT-I tumors of the spine.
CASE REPORT
A 30-year-old male presented with symptoms of hypoesthesia and dysesthesia of the L5 nerve root distribution, with right positive Lasègue, and normal strength and reflexes for 60 days. The patient presented no comorbidities, except the previous treatment of a testicular seminoma with orchiectomy and adjuvant RT 20 years ago. The radiation dose utilized for the treatment of the seminoma was 30.6 Gy, irradiating the cervical and para-aortic regions. There was no personal or family history of neurofibromatosis. He had undergone coronal, sagittal, and axial T2-weighted contrast-enhanced magnetic resonance imaging (MRI) of the lumbar spine that revealed a contrast enhancement of two nodular formations placed in the L4 nerve root, the first located proximally, measuring 1.1 cm × 0.6 cm, and the second distally in the right lateral recess of the spinal canal, measuring 2.1 cm × 0.9 cm [
Figure 1
Coronal, sagittal, and axial T2-weighted contrast-enhanced magnetic resonance imaging of the spine that revealed a contrast-enhancement of two nodular formations, one in the L4 nerve root and the second distally in the right lateral recess of the spinal canal (a). After 6 months, the lumbar magnetic resonance imaging showed that a tumor of the nerve root had grown (b)
The results of the electroneuromyography of lower limbs revealed subacute and chronic right L5 neuropathy without muscle denervation. The genetic analysis was normal. The brain and cervical spine MRI were normal [
Figure 2
The brain and cervical spine magnetic resonance imaging were normal at the first investigation (a). After 7 months sagittal T1-weighted contrast-enhanced magnetic resonance imaging of brain and spine demonstrated a hypertensive hydrocephalus, leptomeningeal enhancement, and contrast-enhanced masses throughout the cervicothoracic spinal cord surfaces (b)
A surgical indication for diagnosis and treatment as well its complications were discussed with the patient and his family. The patient was afraid of motor deficit complications and decided to control the pain with physiotherapy and oral analgesic medication. The patient was closely observed. The pain was well-controlled with physiotherapy and oral analgesia when necessary during 6 months. After this period, the patient complained of a progressive increase of sciatica, the paresthesia became worse, and the motor strength was quickly and severely reduced with Grade 2 dorsiflexion of the right foot strength. The lumbar MRI showed that a tumor of the nerve root had grown [
Surgery was indicated and accepted by the patient and his family. The surgical planning was total tumor removal with free margins. A right hemilaminectomy from L3 to S1 was performed. After the incision of the dura mater, a fusiform tumor was visualized adjacent to the L5 foramen and adjacent of the cauda equina nerve roots. The L4 nerve root was individualized and sectioned proximal to the upper margin of the tumor. The distal tumor was dissected, and the nerve root distally to the tumor was individualized and sectioned. The entire segment of L4 nerve root was resected with clear margins [
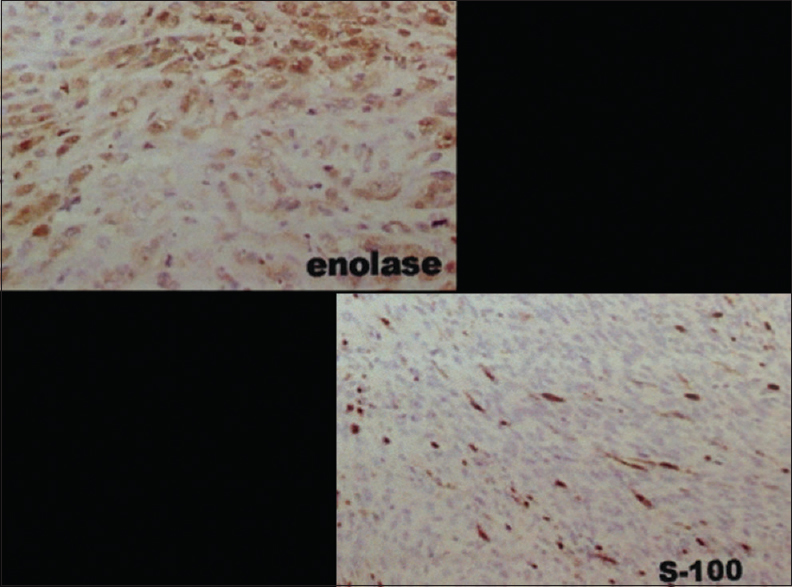
The patient was discharged from hospital 3 days after surgery with better resolution of pain, hypoesthesia in the L5 dermatomes, and motor strength Grade 3 of the L5 nerve root. The anatomopathological and immunohistochemical studies showed a hypercellular malignant spindle cell tumor with a high mitotic index and moderate pleomorphism in a nerve root, compatible with a malignant peripheral nerve sheath tumor (MPNST) [
Thirty days later the patient reported somnolence, anisocoria, diplopia, severe Grade 2 paraparesis, neurogenic bowel with flaccid anal sphincter tone, and neurogenic bladder. Sagittal T1-weighted contrast-enhanced MRI of brain and spine demonstrated a hypertensive hydrocephalus, leptomeningeal enhancement, and contrast-enhanced masses throughout the cervicothoracic spinal cord surfaces [
The patient died from acute respiratory failure 48 h after the second surgery. The patient's total time of survival from the time of MPNST diagnosis was 9 months.
DISCUSSION
Cahan and Woodard.[
The case reported had undergone adjuvant RT for testicular seminoma 20 years before presenting with an RT-I intradural MPNST that developed diffuse leptomeningeal, hydrocephalus, and cervicothoracic spinal cord metastases. A literature review of RT-I spine tumor cases was performed in an online database: pubmed.gov (
According to Toland et al.[
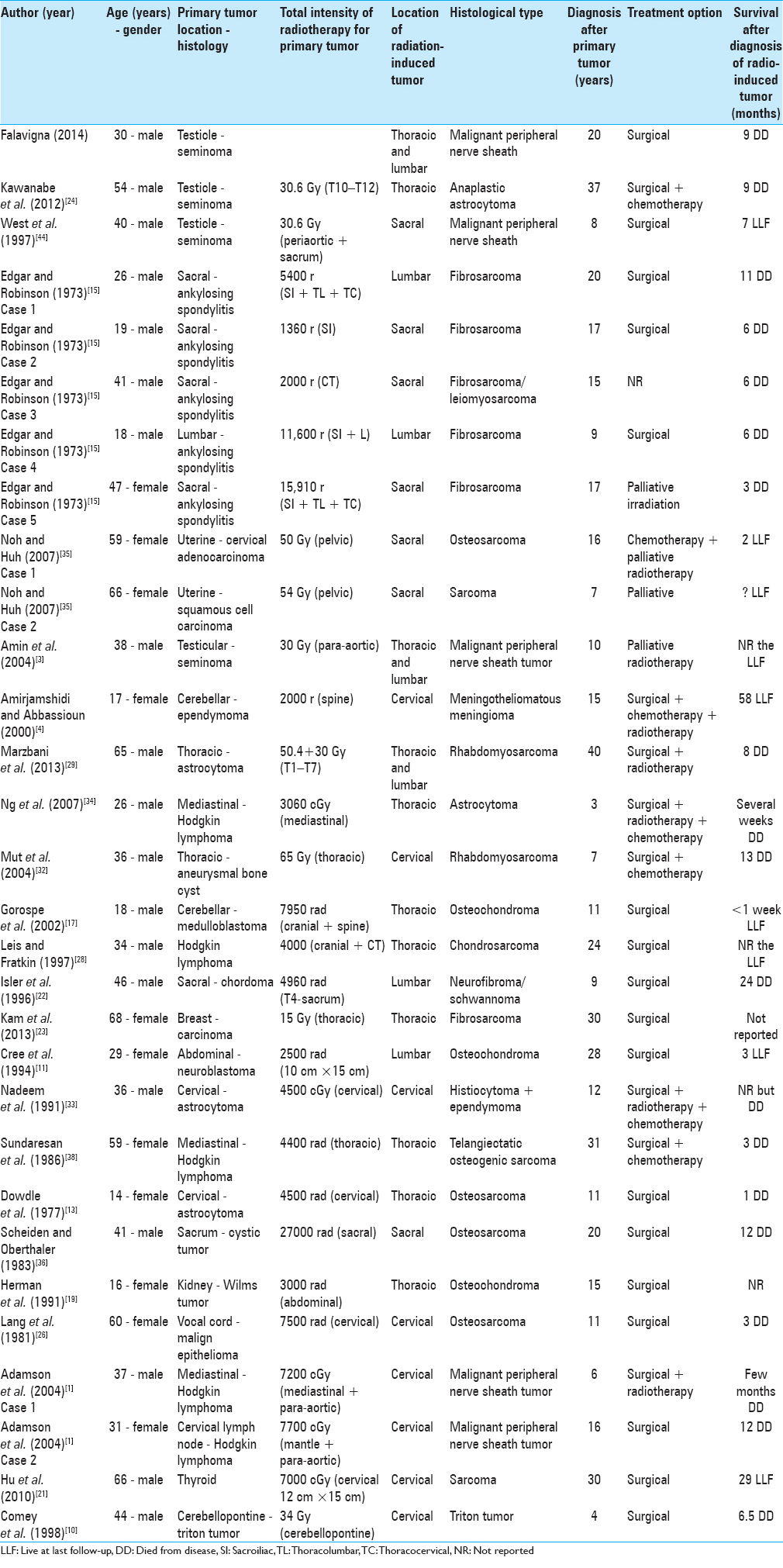
The pathology of the primary tumor of the 30 spine tumors cases induced by RT was mainly neural tumors (30%), followed by Hodgkin's lymphoma (16.6%), ankylosing spondylitis (16.6%), seminoma (13.3%), and astrocytoma (10.0%) [
Prompt recognition and treatment of radio-induced tumors located in the spine are crucial because they tend to grow rapidly and have a low response to conventional radiation and chemotherapy.[
In the reported case, the MRI diagnosis was two small nodular lesions in peripheral nerve compatible with a benign tumor of nerve sheath. This radiological presentation was concordant with the symptoms, and the neurophysiologic study of lower limbs. The genetic analysis was performed to evaluate the presence of neurofibromatosis with a normal result. To identify others lesions in the neuroaxis, brain, cervical, and thoracic MRI were indicated and did not show any tumor except those in the lumbar region. At that time, the patient decides to treat the pain and be closely observed because he was afraid of the chance of residual motor deficit from surgery.
The cases of RT-I tumors reported in the literature were diagnosed on average 16.6 (± 9.73) years after the RT (minimum 3 – maximum 40) [
The most common type of RT-I tumor in the spine was fibrosarcoma (20.0%) followed mainly by MPNST (16.6%), osteosarcoma (13.3%), and osteochondroma (10.0%) [
Surgery is the main therapy in these cases (56.7%), especially because no clear survival benefit has been demonstrated with RT or chemotherapy [
In patients with a history of previous RT that had a clinical presentation of the spine and/or spinal cord dysfunction and where a lesion is detected, a biopsy should be performed to rule out a second primary neoplasm.[
The overall prognosis for patients with secondary RT-I tumors in the spine is dismal because there are no proven benefits of chemotherapy, RT, intraoperative electron irradiation, and brachytherapy, with a 5-year survival rate of around 20%.[
CONCLUSION
RT-I tumors of the spine, peripheral nerve, and spinal cord are not common. Twenty-nine cases have been reported in the literature. Better awareness of this entity and its potential for metastasis to the central nervous system may enable physicians to perform early intervention. The current gold standard therapy is complete resection with clear margins, since most tumors do not respond to chemotherapy and RT. The neural type of RT-I tumor presented a longer survival.
Financial support and sponsorship
The authors received financial support from AOSpine Latin America.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank AOSpine Latin America for the financial support to our study.
References
1. Adamson DC, Cummings TJ, Friedman AH. Malignant peripheral nerve sheath tumor of the spine after radiation therapy for Hodgkin's lymphoma. Clin Neuropathol. 2004. 23: 245-55
2. Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K. EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011. 60: 304-19
3. Amin A, Saifuddin A, Flanagan A, Patterson D, Lehovsky J. Radiotherapy-induced malignant peripheral nerve sheath tumor of the cauda equina. Spine (Phila Pa 1976). 2004. 29: E506-9
4. Amirjamshidi A, Abbassioun K. Radiation-induced tumors of the central nervous system occurring in childhood and adolescence. Four unusual lesions in three patients and a review of the literature. Childs Nerv Syst. 2000. 16: 390-7
5. Baehring JM, Betensky RA, Batchelor TT. Malignant peripheral nerve sheath tumor: The clinical spectrum and outcome of treatment. Neurology. 2003. 61: 696-8
6. Beck A. About this issue, roentgen sarcomas also contribute to the pathogenesis of sarcoma. Muench Med Wonchensehr. 1922. 69: 623-5
7. Cahan WG, Woodard HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948. 1: 3-29
8. Canellos GP, Arseneau JC, DeVita VT, Whang-Peng J, Johnson RE. Second malignancies complicating Hodgkin's disease in remission. Lancet. 1975. 1: 947-9
9. Coleman CN. Secondary neoplasms in patients treated for cancer: Etiology and perspective. Radiat Res. 1982. 92: 188-200
10. Comey CH, McLaughlin MR, Jho HD, Martinez AJ, Lunsford LD. Death from a malignant cerebellopontine angle triton tumor despite stereotactic radiosurgery. Case report. J Neurosurg. 1998. 89: 653-8
11. Cree AK, Hadlow AT, Taylor TK, Chapman GK. Radiation-induced osteochondroma in the lumbar spine. Spine (Phila Pa 1976). 1994. 19: 376-9
12. Davies AM, Sundaram M, James SLJ.editors. Diagnostic Imaging in: Imaging of Bone Tumors and Tumor-Like Lesions: Techniques and Applications, Techniques and Applications. Berlin, Germany: Springer; 2009. p. 503-
13. Dowdle JA, Winter RB, Dehner LP. Postradiation osteosarcoma of the cervical spine in childhood.A case report. J Bone Joint Surg Am. 1977. 59: 969-71
14. Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors.A clinicopathologic study of 120 cases. Cancer. 1986. 57: 2006-21
15. Edgar MA, Robinson MP. Post-radiation sarcoma in ankylosing spondylitis. A report of five cases. J Bone Joint Surg Br. 1973. 55: 183-8
16. Ferguson DJ, Sutton HG, Dawson PJ. Late effects of adjuvant radiotherapy for breast cancer. Cancer. 1984. 54: 2319-23
17. Gorospe L, Madrid-Muñiz C, Royo A, García-Raya P, Alvarez-Ruiz F, López-Barea F. Radiation-induced osteochondroma of the T4 vertebra causing spinal cord compression. Eur Radiol. 2002. 12: 844-8
18. Halperin EC, Greenberg MS, Suit HD. Sarcoma of bone and soft tissue following treatment of Hodgkin's disease. Cancer. 1984. 53: 232-6
19. Herman TE, McAlister WH, Rosenthal D, Dehner LP. Case report 691.Radiation-induced osteochondromas (RIO) arising from the neural arch and producing compression of the spinal cord. Skeletal Radiol. 1991. 20: 472-6
20. Hruban RH, Shiu MH, Senie RT, Woodruff JM. Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer. 1990. 66: 1253-65
21. Hu CK, Kuo LT, Hong WC, Lee JC, Tu YK. Sarcoma of the cervical spine after radiation treatment for thyroid cancer. Spine (Phila Pa 1976). 2010. 35: E363-7
22. Isler MH, Fogaça MF, Mankin HJ. Radiation induced malignant schwannoma arising in a neurofibroma. Clin Orthop Relat Res. 1996. 325: 251-5
23. Kam LS, Anthony MP, Shek H. Radiation-induced sarcoma in spine. Pol J Radiol. 2013. 78: 69-71
24. Kawanabe Y, Sawada M, Yukawa H, Ueda S, Sasaki N, Koizumi T. Radiation-induced spinal cord anaplastic astrocytoma subsequent to radiotherapy for testicular seminoma. Neurol Med Chir (Tokyo). 2012. 52: 675-8
25. Kourea HP, Bilsky MH, Leung DH, Lewis JJ, Woodruff JM. Subdiaphragmatic and intrathoracic paraspinal malignant peripheral nerve sheath tumors: A clinicopathologic study of 25 patients and 26 tumors. Cancer. 1998. 82: 2191-203
26. Lang G, Kehr P, Paternotte H, Aebi J. Radiation-induced sarcoma of the sixth cervical vertebra (author's transl). Rev Chir Orthop Reparatrice Appar Mot. 1981. 67: 691-3
27. Laskin WB, Silverman TA, Enzinger FM. Postradiation soft tissue sarcomas.An analysis of 53 cases. Cancer. 1988. 62: 2330-40
28. Leis AA, Fratkin J. Chondrosarcoma of the spine and thyroid carcinoma following radiation therapy for Hodgkin's lymphoma. Neurology. 1997. 48: 1710-2
29. Marzbani E, Jones RL, Fink J, Chamberlain MC. Delayed development of a rhabdomyosarcoma following radiation for a spinal cord glioma. J Neurooncol. 2013. 112: 115-8
30. Mead GM, Fossa SD, Oliver RT, Joffe JK, Huddart RA, Roberts JT. Randomized trials in 2466 patients with stage I seminoma: Patterns of relapse and follow-up. J Natl Cancer Inst. 2011. 103: 241-9
31. Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA. NCCN clinical practice guidelines in oncology: Testicular cancer. J Natl Compr Canc Netw. 2009. 7: 672-93
32. Mut M, Cataltepe O, Söylemezoglu F, Akalan N, Ozgen T. Radiation-induced malignant triton tumor associated with severe spinal cord compression. Case report and review of the literature. J Neurosurg. 2004. 100: 298-302
33. Nadeem SQ, Feun LG, Bruce-Gregorios JH, Green B. Post radiation sarcoma (malignant fibrous histiocytoma) of the cervical spine following ependymoma (a case report). J Neurooncol. 1991. 11: 263-8
34. Ng C, Fairhall J, Rathmalgoda C, Stening W, Smee R. Spinal cord glioblastoma multiforme induced by radiation after treatment for Hodgkin disease. Case report. J Neurosurg Spine. 2007. 6: 364-7
35. Noh JM, Huh SJ. Two cases of post-radiation osteosarcoma of the sacrum after pelvic irradiation for uterine cervical cancer. Eur J Gynaecol Oncol. 2007. 28: 497-500
36. Scheiden R, Oberthaler W. Radiation induced osteosarcoma of the sacrum following radiation of an undiagnosed bone lesion. Arch Orthop Trauma Surg. 1983. 102: 128-30
37. Sim FH, Cupps RE, Dahlin DC, Ivins JC. Postradiation sarcoma of bone. J Bone Joint Surg Am. 1972. 54: 1479-89
38. Sundaresan N, Huvos AG, Rosen G, Lane JM. Postradiation osteosarcoma of the spine following treatment of Hodgkin's disease. Spine (Phila Pa 1976). 1986. 11: 90-2
39. Toland DH, Coltman CA, Moon TE. Second malignancies complicating Hodgkin's disease: The Southwest Oncology Group Experience. Cancer Clin. 1978. 1: 27-33
40. Tsang RW, Laperriere NJ, Simpson WJ, Brierley J, Panzarella T, Smyth HS. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993. 72: 2227-33
41. Valdueza JM, Hagel C, Westphal M, Hänsel M, Herrmann HD. Primary spinal malignant schwannoma: Clinical, histological and cytogenetic findings. Neurosurg Rev. 1991. 14: 283-91
42. van den Belt-Dusebout AW, de Wit R, Gietema JA, Horenblas S, Louwman MW, Ribot JG. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007. 25: 4370-8
43. van Leeuwen FE, Stiggelbout AM, van den Belt-Dusebout AW, Noyon R, Eliel MR, van Kerkhoff EH. Second cancer risk following testicular cancer: A follow-up study of 1,909 patients. J Clin Oncol. 1993. 11: 415-24
44. West DA, Parra RO, Manepalli A, Bernardi RJ, Cummings JM. Development of a malignant peripheral nerve sheath tumor following treatment for testicular seminoma. Urology. 1997. 50: 292-4
45. Wong WW, Hirose T, Scheithauer BW, Schild SE, Gunderson LL. Malignant peripheral nerve sheath tumor: Analysis of treatment outcome. Int J Radiat Oncol Biol Phys. 1998. 42: 351-60