- Department of Neurosurgery, Highly Specialized Hospital and of National Importance “Garibaldi”, Catania, Italy,
- Department of Neurosurgery, Cannizzaro Hospital, “Garibaldi”, Catania, Sicily, Italy.
- Department of Clinical Pathology and Molecular Biology Unit, Highly Specialized Hospital of National Importance “Garibaldi”, Catania, Sicily, Italy.
Correspondence Address:
Gianluca Scalia
Department of Neurosurgery, Highly Specialized Hospital and of National Importance “Garibaldi”, Catania, Italy,
DOI:10.25259/SNI_525_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Massimo Furnari1, Gianluca Scalia1, Giuseppe Emmanuele Umana2, Massimiliano Giuffrida1, Giancarlo Ponzo1, Sebastiano Fabio Garozzo3, Giovanni Federico Nicoletti1. Rare spondylodiscitis due to Mycobacterium mucogenicum. 12-Sep-2020;11:289
How to cite this URL: Massimo Furnari1, Gianluca Scalia1, Giuseppe Emmanuele Umana2, Massimiliano Giuffrida1, Giancarlo Ponzo1, Sebastiano Fabio Garozzo3, Giovanni Federico Nicoletti1. Rare spondylodiscitis due to Mycobacterium mucogenicum. 12-Sep-2020;11:289. Available from: https://surgicalneurologyint.com/surgicalint-articles/10251/
Abstract
Background: Nontuberculous mycobacteria (NTM) represents an important cause of infection, particularly in immunocompromised patients. Spondylodiscitis is unusual and may be associated with underlying causes such as drug abuse. Timely diagnosis and treatment are critical, as without this, patients will demonstrate progressive neurological deterioration. Here, we present a rare case of Mycobacterium mucogenicum spondylodiscitis in a 36-year-old male, along with a focused literature review.
Case Description: A 36-year-old female with previous drug abuse presented with 3-years of progressive thoracolumbar pain. The MRI of the spine revealed paravertebral abscesses from Th10–L1 with vertebral lesions involving Th11–Th12 levels (e.g., vertebral body collapse/deformity and destruction of the posterior vertebral walls). After a needle CT-guided biopsy of the paravertebral tissues, real time-polymerase chain reaction (RT-PCR) amplification documented NTM; the final identification was M. mucogenicum. The patient then underwent a Th11–Th12 decompressive laminectomy, facetectomy, granulomatous tissue debridement, and posterior pedicle screw fusion from Th8–Th10, and L1–L3. Postoperatively, the patient’s pain resolved, and she was left with residual lower extremities dysesthesias; 6-months later, she could walk without assistance.
Conclusion: Spondylodiscitis caused by M. mucogenicum is rare, and the medical and surgical treatment is comparable to that for other NTM groups.
Keywords: Mycobacteria, Spondylodiscitis, Deformity, Posterior fixation, Corpectomy
INTRODUCTION
Rapidly growing nontuberculous mycobacteria (NTM) represents an important cause of infection, particularly in immunocompromised patients.[
MATERIALS AND METHODS
Our literature search from PubMed and Scopus databases (Mesh terms: vertebral osteomyelitis OR spondylodiscitis AND mycobacterium chelonae OR M. mucogenicum) revealed six case reports focused on vertebral osteomyelitis/ spondylodiscitis caused by M. mucogenicum subgroup (1993- 2020).[
CASE DESCRIPTION
A 36-year-old female with chronic hepatitis C and previous drug abuse presented with a 3-year history of progressive thoracolumbar pain. On admission, she was febrile, had lower extremities dysesthesias with weakness. The white blood cell count (WBC) was slightly elevated at 12.000/μL, while the erythrocyte sedimentation rate (ESR) (40 mm/h) and a C-reactive protein (CRP) serum levels (49 mg/L) were high.
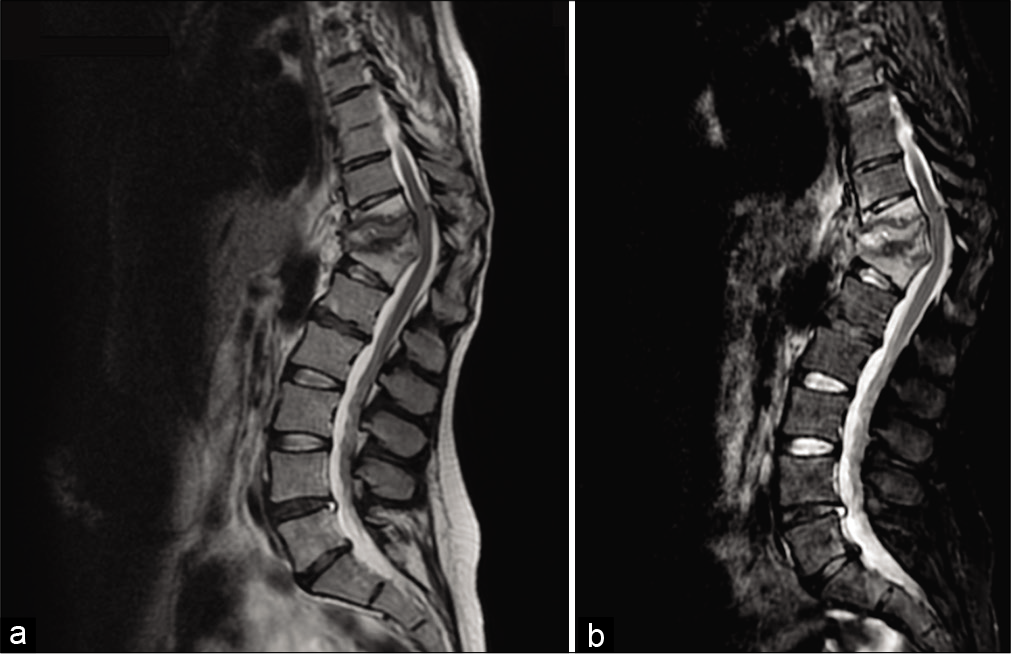
The thoracic MRI showed Th11–Th12 vertebral body collapse, (vertebral deformity with a Th11–Th12 sagittal Cobb angle of 53°) involvement of the posterior vertebral walls, and paravertebral abscesses at Th10–L1 [
Radiological and laboratory diagnosis of NTM
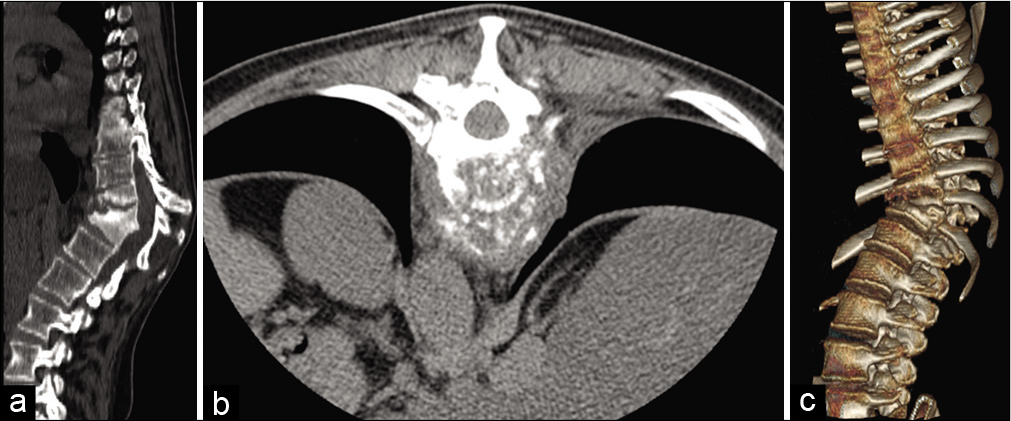
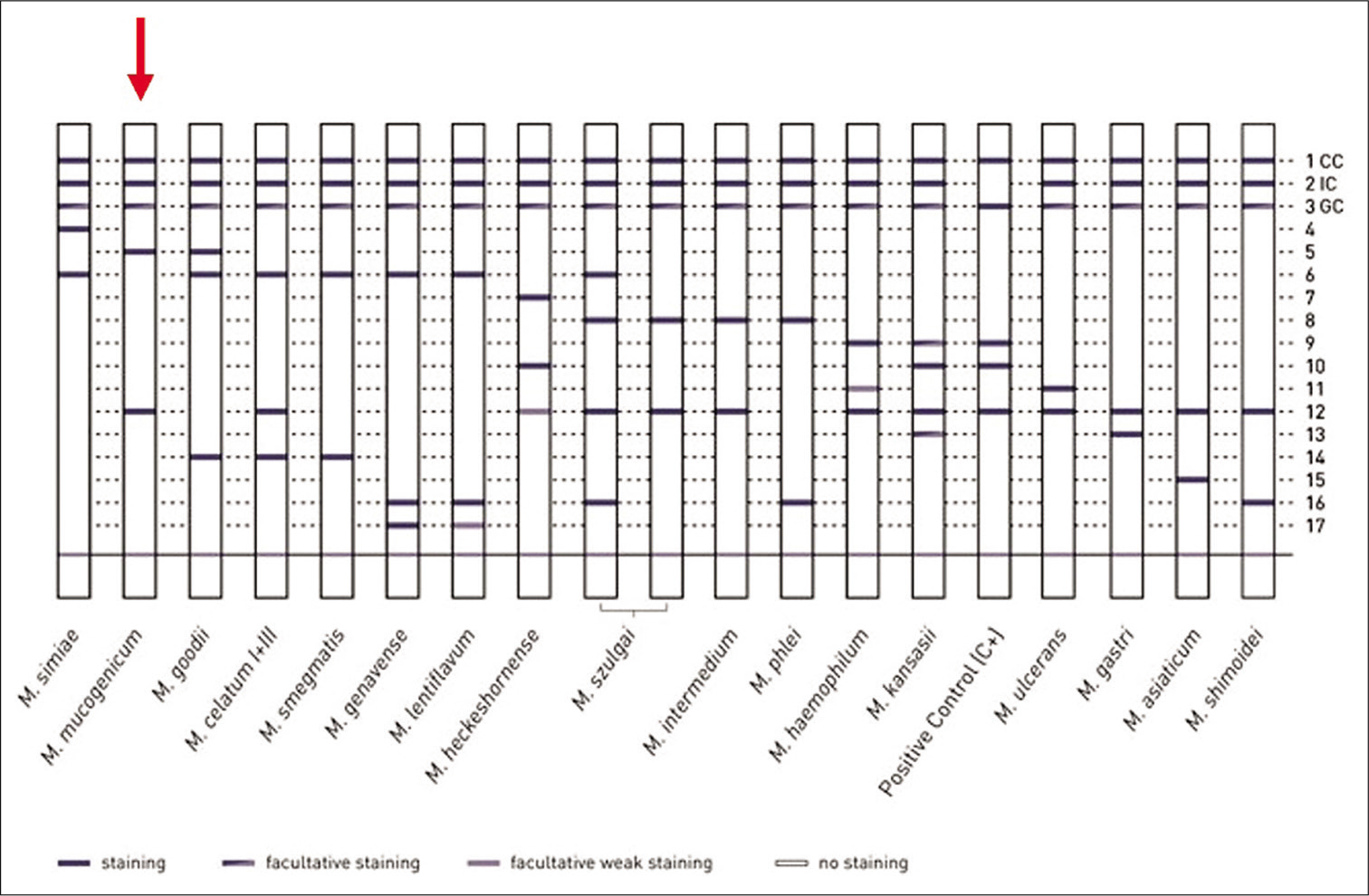
First, a needle-guided CT-guided biopsy was performed of the paravertebral tissues; this revealed fibrofatty tissue with chronic nonspecific inflammation and areas of hemorrhagic dissociation with histiocytes, but the tissue culture was negative. In a second phase, real time-polymerase chain reaction (RT-PCR) amplification showed positive results for NTM. The “Genotype CMdirect” molecular test was carried out [
Surgery
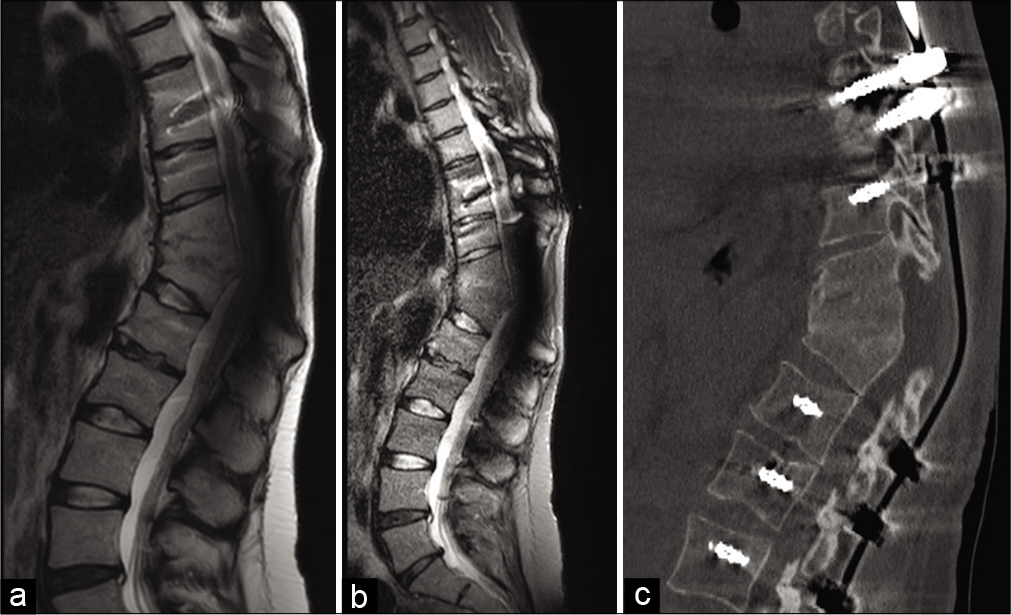
We performed a Th11–Th12 decompressive laminectomy, facetectomy, and granulomatous tissue debridement; a posterior pedicle screw fusion was performed at both the Th8–Th10 and L1–L3. The bacterial specimens confirming the diagnosis of M. mucogenicum. Postoperatively, the patient improved, with gradual alleviation of back pain, and lower extremities dysesthesias. She was continued on medical therapy (e.g., amoxicillin-clavulanic acid 1000/125 mg twice daily) for 3 weeks. At 6-months, she was able to walk without assistance, and the MRI and CT scans confirmed adequate neural decompression/fusion (e.g., complete Th11–Th12 vertebral fusion, with a sagittal Cobb angle of 38°) without recurrence of infection [
DISCUSSION
M. mucogenicum is frequently found in tap water, including ice machines and this can contribute to the transient colonization or contamination of sputum samples.[
In the case of surgical treatment, there is still no uniform indication of the type of approach to choose, as anterior, posterior, combined in a single stage or deferred. Some authors stated that the advantages of a posterior approach include beyond decompression of nerve structures, correction of spinal deformity, reduced surgical times, less blood loss, reduced postoperative pain, less hospital stay, and overall a better quality of life for the patient.[
CONCLUSION
Spondylodiscitis caused by M. mucogenicum is rare and, here, was appropriately treated with posterior decompression and fusion.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Adékambi T. Mycobacterium mucogenicum group infections: A review. Clin Microbiol Infect. 2009. 15: 911-918
2. Korres DS, Papagelopoulos PJ, Zahos KA, Kolia MD, Poulakou GG, Falagas ME. Multifocal spinal and extra-spinal Mycobacterium chelonae osteomyelitis in a renal transplant recipient. Transpl Infect Dis. 2007. 9: 62-5
3. Marino A, Caltabiano E, Zagami A, Onorante A, Zappalà C, Locatelli ME. Rapid emergence of cryptococcal fungemia, Mycobacterium chelonae vertebral osteomyelitis and gastrointestinal stromal tumor in a young HIV late presenter: A case report. BMC Infect Dis. 2018. 18: 693
4. Metta H, Corti M, Brunzini R. Disseminated infection due to Mycobacterium chelonae with scleritis, spondylodiscitis and spinal epidural abscess. Braz J Infect Dis. 2008. 12: 260-2
5. Pruitt TC, Hughes LO, Blasier RD, McCarthy RE, Glasier CM, Roloson GJ. Atypical mycobacterial vertebral osteomyelitis in a steroid-dependent adolescent. A case report. Spine (Phila Pa 1976). 1993. 18: 2553-5
6. Rahman I, Bhatt H, Chillag S, Duffus W. Mycobacterium chelonae vertebral osteomyelitis. S Med J. 2009. 102: 1167-9
7. Tortoli E, Mantella A, Mariottini A, Mazzarelli G, Pecile P, Rogasi PG. Successfully treated spondylodiscitis due to a previously unreported mycobacterium. J Med Microbiol. 2006. 55: 119-21
8. Wang X, Pang X, Wu P, Luo C, Shen X. One-stage anterior debridement, bone grafting and posterior instrumentation vs. single posterior debridement, bone grafting, and instrumentation for the treatment of thoracic and lumbar spinal tuberculosis. Eur Spine J. 2014. 23: 830-7