- Department of Neurosurgery, Memfys Hospital, Transekulu, Enugu, Nigeria,
- Department of Neurosurgery, Shinshu University Hospital, Matsumoto, Japan
- Department of Neurosurgery, Japanese Red Cross Society Suwa Hospital, Suwa, Japan
- Department of Neurosurgery, Shinshu University School of Medicine, Matsumoto, Japan.
Correspondence Address:
Kohei Kanaya, Department of Neurosurgery, Shinshu University Hospital, Matsumoto, Japan.
DOI:10.25259/SNI_978_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Campbell Chukwuebuka Francis1, Kohei Kanaya2, Kohei Nagamine2, Tetsuya Goto3, Tetsuyoshi Horiuchi4, Samuel Chukwunonyerem Ohaegbulam1. Rare vermian pilocytic astrocytoma with recurrent spontaneous hemorrhage in the elderly: A case report and review of literature. 15-Mar-2024;15:90
How to cite this URL: Campbell Chukwuebuka Francis1, Kohei Kanaya2, Kohei Nagamine2, Tetsuya Goto3, Tetsuyoshi Horiuchi4, Samuel Chukwunonyerem Ohaegbulam1. Rare vermian pilocytic astrocytoma with recurrent spontaneous hemorrhage in the elderly: A case report and review of literature. 15-Mar-2024;15:90. Available from: https://surgicalneurologyint.com/surgicalint-articles/12799/
Abstract
Background: Pilocytic astrocytoma (PA) is a benign glial tumor predominately seen in pediatrics and early adolescence with associated overall good outcomes. Very few cases of elderly PA have been reported in the literature, and they are known to display unique anatomic, histologic, and genetic peculiarities distinct from pediatric disease. We report a rare case of vermian PA in an octogenarian with recurrent spontaneous intratumoral hemorrhage as a presenting symptom. Furthermore, a review of the literature on the peculiarities of PA in the elderly will be discussed.
Case Description: An 81-year-old woman presented with features suggestive of repeated posterior fossa hemorrhages characterized by headaches, diplopia, and alteration in sensorium occurring about 5 months apart. Brain neuroimaging showed a cerebellar vermian tumor with features suggestive of repeated intratumoral bleeding. She had an initial ventriculoperitoneal shunting for acute hydrocephalus and subsequently had a suboccipital craniotomy and subtotal tumor excision due to morbid adherence to the brainstem. The histologic diagnosis was PA with Ki-67
Conclusion: PA in the elderly is a rare disease with distinct histologic and genetic peculiarities. This case review showed one of the oldest cases of cerebellar vermian PA presenting with recurrent spontaneous intratumoral hemorrhage, an extremely rare occurrence in benign glioma. Although complete surgical excision is recommended, partial resection is advocated for morbidly adherent tumors. Overall prognosis is worse in elderly PA.
Keywords: Elder, Intratumoral hemorrhage, Pilocytic astrocytoma, Vermian tumor
INTRODUCTION
Pilocytic astrocytoma (PA) is the most common primary central nervous system (CNS) glial neoplasm in childhood and early adolescence.[
Clinical presentation depends on anatomic location and the associated mass effect. Although spontaneous intratumoral hemorrhages occur in malignant gliomas and metastatic brain tumors, they are unusual in PA and rarely reported in the elderly. Mechanisms of apoplectiform tumor bleeding have been attributed to abnormal neovascularization within the tumor and adjacent brain invasion.[
We present a rare case of vermian PA in an octogenarian who presented with recurrent hemorrhage and hydrocephalus. Furthermore, a review of the literature on the peculiarities of PA in the elderly will be discussed.
CASE PRESENTATION
An 81-year-old elderly woman presented with cognitive decline characterized by memory impairment and behavioral changes, which progressively worsened with time. She subsequently developed sudden onset headache and rapid deterioration of her clinical condition, necessitating presentation to the hospital. There was no history suggestive of neurofibromatosis type-1. She has no comorbidities but had treatment for colon cancer about 7 years ago without evidence of recurrence.
Examination findings showed a confused lady with a Glasgow Coma Scale score of E4V4M6. The speech was fluent, and the Mini–Mental State Examination was 13/30 (suggestive of moderate to severe dementia). Her Karnofsky performance score was 60%. She has no meningeal signs, and cranial nerve examinations were essentially normal. Long tracts were normal, and no abnormal reflexes were elicited. However, she has an unstable gait and truncal ataxia. Finger nose testing was normal, and dysdiadochokinesia was absent.
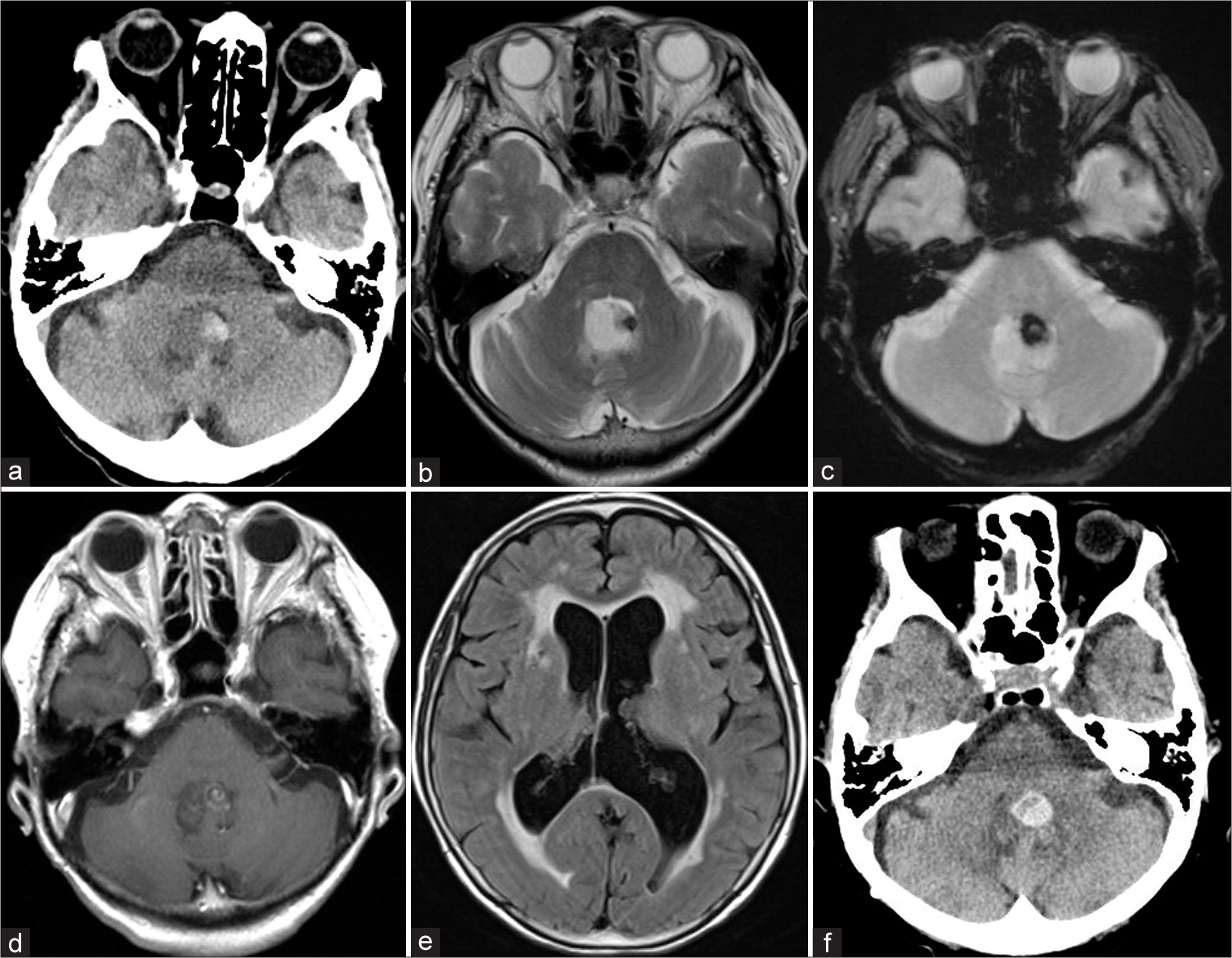
Her cranial computed tomography scan showed a solid vermian hyperdense mass with intratumoral hemorrhage [
Figure 1:
(a-c) Initial computed tomography (CT) scan and T2- and T2-star-weighted images showing a vermian mass lesion with associated intratumoral hemorrhage. (d) Enhanced T1-weighted image showing minimal enhancement of the tumor. (e) Fluid-attenuated inversion recovery image showing hydrocephalus. (f) CT scan 5 months later showing a repeat spontaneous intratumoral hemorrhage.
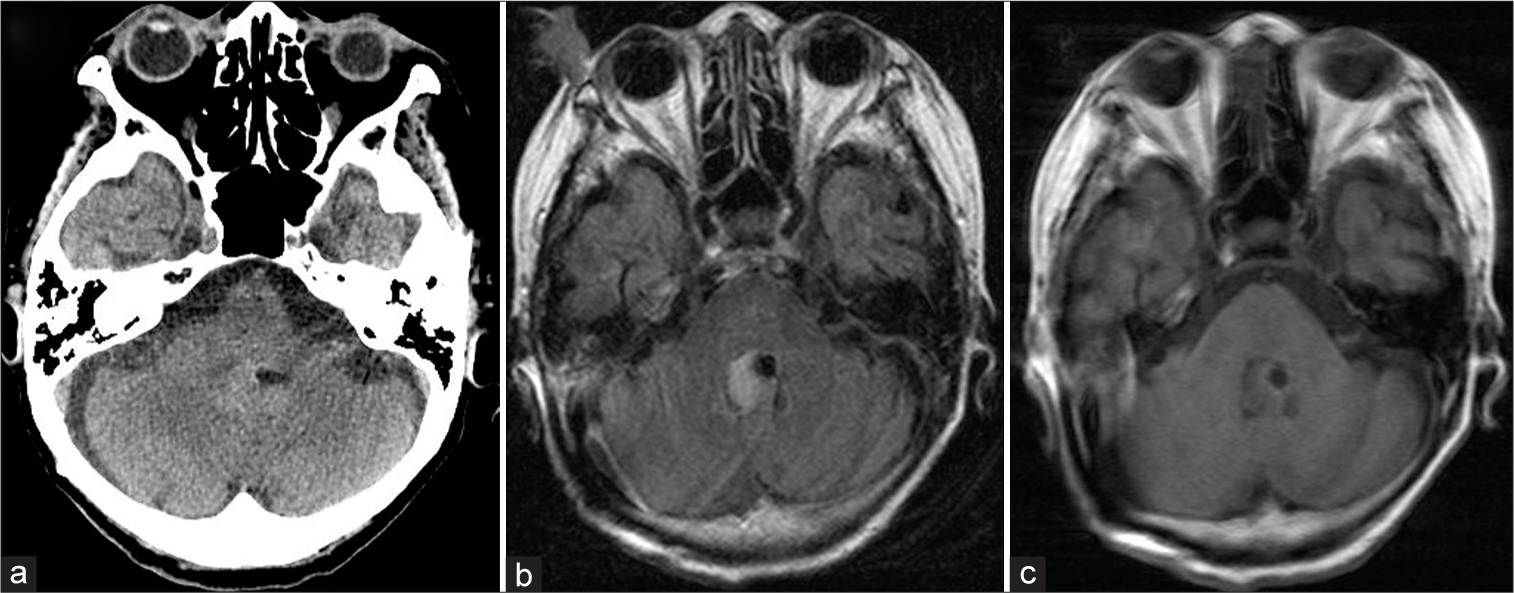
Five months later, she developed acute worsening symptoms with an associated alteration in consciousness, diplopia, florid cerebellar symptoms, and gait instability. Repeat neuroimaging showed rebleeding of the vermian lesion with features suggestive of posterior fossa hypertension [
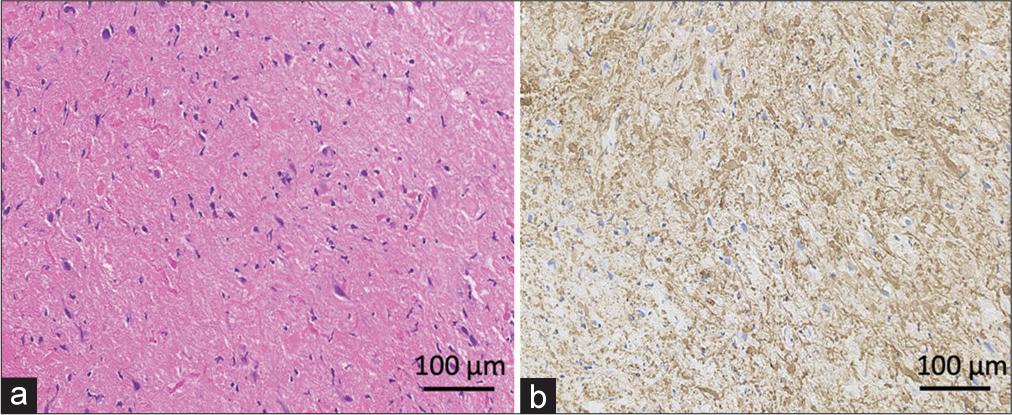
Histology showed densely packed atypical glial lineage cells, with Rosenthal fibers (RFs) and eosinophilic granular bodies (EGBs) scattered in the background. Only a few mitotic figures were seen. The features were consistent with PA [
DISCUSSION
PA was first described by Harvey Cushings in 1931 as a distinct tumor type found in the cerebellum with a better prognosis when compared to supratentorial glioma.[
Although pediatric PA has an excellent prognosis, the disease in the elderly may have different biological behavior and comparatively worse outcomes.[
Epidemiology of PA in the elderly
PA is essentially a pediatric disease constituting about 30–40% of childhood brain tumors with predominant posterior fossa localization.[
Unlike pediatric PA, most elderly cases involve the supratentorial compartment and display slight female preponderance.[
Histopathology of PA
PA has biphasic histologic architecture characterized by loose microcystic areas containing astroglial cells and EGB with hyaline droplets and more solid components displaying increased cellularity and focal accumulation of RF.[
Furthermore, features of cellular degeneration have also been described, including increased cellularity and mitosis, nuclear atypia, psammomatous calcification, necrosis, and endovascular proliferation.[
Immunohistochemistry is pivotal to typing these tumors correctly. They show typical astroglial lineage features, including strong positivity for GFAP and stain for S-100 protein and OLIG2. Ki-67 proliferative index is usually low except in cases of malignant transformation. Although this index case did not have the typical bipolar features, they had numerous RF and EGB in the compact background with positive stains for GFAB. In addition, KI-67 was low, and P53 gene and IDH-1 mutations were absent.[
Specific characteristic mutations seen in other gliomas are absent in PA; only two isolated case reports of IDH-1 mutation have been reported in PA, and all were in elderly patients.[
Molecular peculiarities of PA
Pediatric astrocytoma was described as a one-pathway disease because more than 90% involve dysregulation of the mitogen-activated protein kinase (MAPK) pathway essential for cell proliferation and neurogenesis.[
The BRAF fusion involves duplication of chromosome 7q34 encompassing the BRAF gene resulting in the fusion of the N-terminal of KIAA1549 protein to the regulatory region of BRAF and attendant unregulated constitutional activity of the MAPK pathway.[
Indeed, since elderly PA are extremely rare, and no large-scale characterization of the genetic abnormalities has been reported, it is assumed that they harbor similar mutations to adult PA. In addition, other rare genetic abnormalities have also been observed in elderly PA, including IDH R132H mutation, ATRX alteration, and CDKN2A loss.[
Clinical presentation and diagnosis
Clinical features of PA are related to the anatomic location and associated mass effect. Cerebellar lesions usually present with gait disturbances, coordination disorders, and hydrocephalus. Although reports of intratumoral bleeding are seen in glioblastoma and metastatic diseases, its occurrence in benign intracranial tumors is rare.[
The etiology of bleeding in intracranial tumors is unclear. Neovascularization and endothelial proliferation within the tumor may be contributory, with the nascent vessels lacking elastic fibers and extracellular matrix support.[
Brain MRI is the diagnostic imaging tool of choice, and PA may be solid, cystic, or a combination of both. The most common neuroimaging finding in PA is a cystic lesion with an enhancing mural nodule, also seen in hemangioblastoma, cystic metastasis, ganglioglioma, and pleomorphic xanthoastrocytoma.[
Management options
Surgical excision is the primary treatment strategy for PA and results in excellent outcomes with 5-year progression-free survival of more than 95% in pediatrics.[
Some authors opined that excision of the mural nodule suffices as it contains the neoplastic glioma cells, and the cyst wall should be left in situ.[
Adjuvant radiotherapy is advocated for disease recurrence, especially in poor surgical candidates; nevertheless, repeat surgery should be considered in all cases.[
Care is multidisciplinary, involving the neurosurgeon, physician, radiologist, histopathologist, and rehabilitation expert. Counseling on recurrence risk and management options is essential before treatment and the need for long-term follow-up.
CONCLUSION
PA is a common neoplastic glial tumor in pediatric and early adolescence but is rarely reported in the elderly population. Specific anatomic, histologic, and genetic peculiarities make elderly PA unique and distinct from pediatric disease and confer higher recurrence risk and poorer outcomes. This case review showed one of the oldest cases of cerebellar vermian PA presenting with recurrent spontaneous intratumoral hemorrhage, an unusual occurrence in benign glioma. Although complete surgical excision is recommended, partial excision is advocated for morbidly adherent tumors.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
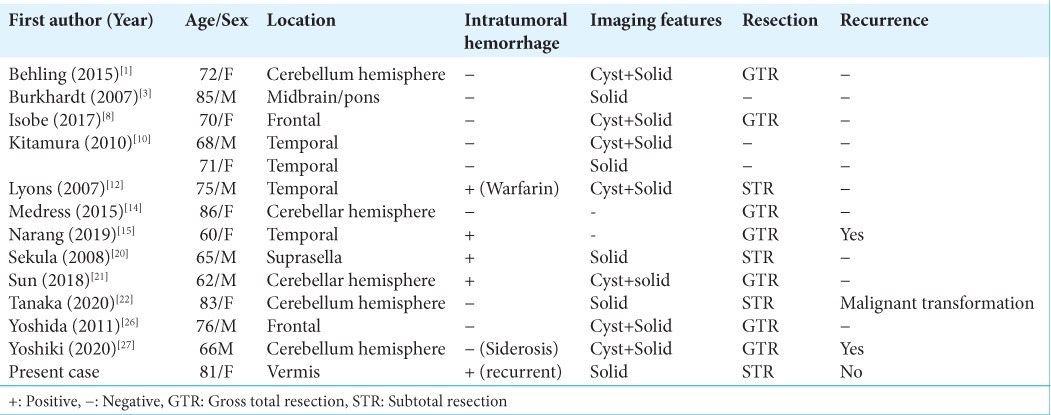
1. Behling F, Steinhilber J, Tatagiba M, Bisdas S, Schittenhelm J. IDH1 R132H mutation in a pilocytic astrocytoma: A case report. Int J Clin Exp Pathol. 2015. 8: 11809-13
2. Beni-Adani L, Gomori M, Spektor S, Constantini S. Cyst wall enhancement in pilocytic astrocytoma: Neoplastic or reactive phenomena. Pediatr Neurosurg. 2000. 32: 234-9
3. Burkhardt K, Heuberger F, Delavelle J. Pilocytic astrocytoma in the elderly. Clin Neuropathol. 2007. 26: 306-10
4. Collins VP, Jones DT, Giannini C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015. 129: 775-88
5. Cushing H. Experiences with the cerebellar astrocytomas. Surg Gynecol Obstet. 1931. 52: 129-204
6. Gregory TA, Chumbley LB, Henson JW, Theeler BJ. Adult pilocytic astrocytoma in the molecular era: A comprehensive review. CNS Oncol. 2021. 10: CNS68
7. Hasselblatt M, Riesmeier B, Lechtape B, Brentrup A, Stummer W, Albert FK. BRAF-KIAA1549 fusion transcripts are less frequent in pilocytic astrocytomas diagnosed in adults. Neuropathol Appl Neurobiol. 2011. 37: 803-6
8. Isobe N, Nishimoto T, Ueda T. An elderly patient with pilocytic astrocytoma operated on 14 years after initial detection: A case study. No Shinkei Geka. 2017. 45: 707-13
9. Johnson DR, Brown PD, Galanis E, Hammack JE. Pilocytic astrocytoma survival in adults: Analysis of the surveillance, epidemiology, and end results program of the national cancer institute. J Neurooncol. 2012. 108: 187-93
10. Kitamura N, Hasebe T, Kasai R, Kasuya S, Nakatsuka T. Pilocytic astrocytomas in elderly adults. Neuroradiol J. 2010. 23: 690-5
11. Li HM, Hsu SS, Wang JS, Weng MJ, Fu JH, Chen CK. Cerebral pilocytic astrocytoma with spontaneous intracranial hemorrhage in adults. J Chin Med Assoc. 2008. 71: 587-93
12. Lyons MK. Pilocytic astrocytoma with spontaneous intracranial hemorrhages in an elderly adult. Clin Neurol Neurosurg. 2007. 109: 76-80
13. Matyja E, Grajkowska W, Stȩpień K, Naganska E. Heterogeneity of histopathological presentation of pilocytic astrocytoma-Diagnostic pitfalls. A review. Folia Neuropathol. 2016. 54: 197-211
14. Medress ZA, Xu LW, Ziskin JL, Lefterova MI, Vogel H, Li G. Pilocytic astrocytoma with IDH1 mutation in the cerebellum of an elderly patient. Clin Neuropathol. 2015. 34: 96-8
15. Narang A, Aggarwal V, Kavita D, Maheshwari C, Bansal P. Cerebral pilocytic astrocytoma with spontaneous intratumoral haemorrhage in the elderly-a rare entity: A case report and review of literature. Roman Neurosurg. 2019. 33: 156-9
16. Ostrowski RP, He Z, Pucko EB, Matyja E. Hemorrhage in brain tumor-An unresolved issue. Brain Hemorrhages. 2022. 3: 98-102
17. Pathak P, Kumar A, Jha P, Purkait S, Faruq M, Suri A. Genetic alterations related to BRAF-FGFR genes and dysregulated MAPK/ERK/mTOR signaling in adult pilocytic astrocytoma. Brain Pathol. 2017. 27: 580-9
18. Salles D, Laviola G, de Moraes Malinverni, Stávale JN. Pilocytic astrocytoma: A review of general, clinical, and molecular characteristics. J Child Neurol. 2020. 35: 852-8
19. Santos AN, Dieckmann C, Rauschenbach L, Oppong MD, Dinger TF, Deuschl C. Long-term outcome after management of pilocytic astrocytoma in the posterior fossa in a pediatric population. IBRO Neurosci Rep. 2022. 13: 388-92
20. Sekula RF, Marchan EM, Quigley MR, Frederickson AM, Pu C. A case of an elderly adult presenting with obstructive hydrocephalus secondary to a rare hemorrhagic suprasellar pilocytic astrocytoma. Clin Neuropathol. 2008. 27: 396-9
21. Sun S, Zhou H, Ding ZZ, Shi H. Cerebellar pilocytic astrocytomas with spontaneous intratumoral hemorrhage in the elderly A case report and review of the literature. Medicine (Baltimore). 2018. 97: e11329
22. Tanaka T, Teshigawara A, Takei J, Tochigi S, Hasegawa Y, Murayama Y. Rapid recurrence and anaplastic transformation of a pilocytic astrocytoma in an elderly patient: Case report and review of the literature. World Neurosurg. 2020. 142: 441-9
23. Theeler BJ, Ellezam B, Sadighi ZS, Mehta V, Diep Tran M, Adesina AM. Adult pilocytic astrocytomas: Clinical features and molecular analysis. Neuro Oncol. 2014. 16: 841-7
24. Wade A, Hayhurst C, Amato-Watkins A, Lammie A, Leach P. Cerebellar pilocytic astrocytoma in adults: A management paradigm for a rare tumour. Acta Neurochir (Wien). 2013. 155: 1431-5
25. White JB, Piepgras DG, Scheithauer BW, Parisi JE. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg. 2008. 108: 223-6
26. Yoshida Y, Tsukada T, Hashimoto M, Hayashi Y. Pilocytic astrocytoma of the cerebrum presenting in an elderly patient: A case report. No Shinkei Geka. 2011. 39: 865-9
27. Yoshiki K, Sasagawa Y, Kinoshita M, Furuta T, Tamai S, Sabit H. Superficial siderosis associated with long-term recurrence of pilocytic astrocytoma in an elderly person. World Neurosurg. 2020. 138: 541-4.e1