- Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Science, Tehran, Iran,
- Department of Neurosurgery, Medical Center Saarbruecken, Saarland, Germany,
- Department of Endocrinology, Loghman Hakim Hospital, Shahid Beheshti University of Medical Science, Tehran, Iran.
Correspondence Address:
Mahmoud Lotfinia, Department of Neurosurgery, Medical Center Saarbruecken, Saarland, Germany.
DOI:10.25259/SNI_1096_2021
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Guive Sharifi1, Arsalan Amin1, Mahmoud Lotfinia2, Mohammad Hallajnejad1, Zahra Davoudi3, Nader Akbari Dilmaghani1, Omidvar Rezaei Mirghaed1. Rathke’s cleft cysts: A single-center case series. 19-Aug-2022;13:368
How to cite this URL: Guive Sharifi1, Arsalan Amin1, Mahmoud Lotfinia2, Mohammad Hallajnejad1, Zahra Davoudi3, Nader Akbari Dilmaghani1, Omidvar Rezaei Mirghaed1. Rathke’s cleft cysts: A single-center case series. 19-Aug-2022;13:368. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=11809
Abstract
Background: Rathke’s cleft cysts (RCCs) are common benign sellar or suprasellar lesions. The aim of this study is to report our experience on the management of 27 RCC cases.
Methods: We retrospectively analyzed a series of 27 patients with symptomatic RCC who were referred to our department between January 2016 and January 2020. Data regarding patients’ demographics, clinical evaluations, laboratory and neuroimaging findings, pathologic records, surgical treatment, and complications were extracted from our electronic database. All patients underwent RCC removal through a direct endoscopic endonasal transsphenoidal (EETS) approach, except for two cases.
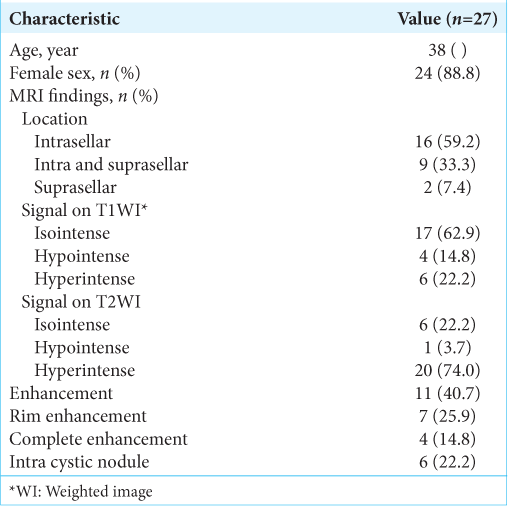
Results: Data of 27 patients (3 men and 24 women; mean age: 38 years) with symptomatic RCC were reviewed. The most common presenting symptom was headache, occurring in 20 (74.0%) patients. In 16 (59.2%) cases, the tumor was primarily located in the sella turcica. Nine (33.3%) cases exhibited a secondary suprasellar extension.
Conclusion: Our experience with RCC patients showed that EETS is a safe method of treatment, with minimal recurrence.
Keywords: Neuroendoscopy, Outcome assessment, Pituitary gland, Rathke’s cleft cyst
INTRODUCTION
Rathke’s cleft cysts (RCCs) are relatively common, benign, nonneoplastic, and intra- and suprasellar lesions originating from the remnant of Rathke’s pouch lesions composed typically of a thin cyst wall enclosing a mucous, gelatinous, or caseous liquid core.[
RCCs are included in the differential diagnosis with other cystic lesions in such regions, such as craniopharyngiomas, arachnoid cysts, epidermoid cysts, and cystic pituitary adenomas and typically are diagnosed based on the shape, signal intensity, and enhancement characteristics of the lesions on magnetic resonance imaging (MRI).[
Several radiological features of RCCs have been described in the literature, but there is a general consensus about the limited sensitivity of each of this imaging findings.[
MATERIALS AND METHODS
Patients
In this study, we retrospectively evaluated records of patients (n = 27) with symptomatic RCC, who were referred to our department between January 2016 and January 2020 and met our inclusion criteria, that is, surgical and histological verification of diagnosis of RCC. We retrieved and reviewed the data regarding preoperative and postoperative clinical manifestations, neurological examination, visual acuity and field, laboratory tests, neuroimaging findings, pathologic records, intervention plans, and complications. The review board and ethics committee of Shahid Beheshti University of Medical Sciences approved the study design. All research was performed in accordance with relevant guidelines and regulations. We contacted the patients and obtained informed consent for the anonymous use of their data in this study. Informed consent was obtained from parent and/or legal guardian of all minor subjects (age under 18).
MRI data acquisition
All MRI studies were performed with a superconducting magnet 1.5-tesla scanner. Before gadolinium injection, T1-weighted SE and T2-weighted turbo SE images, followed by coronal dynamic acquisition (T1-weighted turbo SE), were obtained in the coronal plane using the following protocol: TR/TE, 400/20 ms; 288 • 192 matrix; 2 excitations; 18 • 18 cm field of view; 3 mm in thickness with 0.3-mm intersection gap. Beginning simultaneously with gadolinium injection, coronal and sagittal T1-weighted SE images were obtained 2 min after the injection. The radiologists and surgeons independently reviewed all imaging studies. Unenhanced and enhanced MRI was available for all patients and their reports were retrieved from the databank of the imaging center. MRI features, including location, size, shape, and signal characteristics of the lesion were reviewed. Cyst location and diameter were assessed on the sagittal MRI. We suspected RCC in patients with a midline, homogeneous fluid lesion, with little or no enhancement after GAD injection.
Diagnosis confirmation
The diagnosis was confirmed in each case based on the histological criteria for RCC, that is, dense eosinophilic amorphous mucin containing small strips of simple cuboidal or pseudostratified columnar, ciliated epithelial cyst wall lining. The preoperative and postoperative hormone profile of the patients (prolactin, thyroid function tests, and gonadotropins), as well as the initial clinical manifestations and the effect of surgery on them, were reviewed.
Surgical technique
RCC removal performed through a standard Endoscopic endonasal transsphenoidal (EETS) approach. In this condition and after dural opening, the cyst wall was incised and cyst content was removed by suction if possible. After decompression, the cyst wall was removed as much as it was safe and a gross-total resection was attempted only when it was deemed safe and feasible. Occasionally, the removal involved a direct approach through the anterior-inferior pituitary gland through a low midline vertical glandular incision (i.e., transpituitary approach). At the completion of the cyst removal, the cavity was carefully inspected for residual cyst contents and cyst lining. An angled endoscope was used for assisted visualization in all endoscopic cases. For patients who showed suprasellar extension, we used an extended approach by partial drilling of the pars planum of the sphenoid bone. By this extended transplanum approach, removal of the suprasellar component of the lesion was generally feasible. Cerebrospinal fluid (CSF) leak identified after cyst removal was repaired with fat tissue and collagen sponge. In our experience, there was no need for using fascia lata or other material for preventing CSF leak. For patients without an intraoperative CSF leak, only a collagen sponge was placed.
RESULTS
Twenty-seven patients (three men and 24 women; age range: 9–65 years, mean age: 38 years) with symptomatic RCC were admitted to our department between January 2016 and January 2020. The average follow-up time was 18 months (range: 3–36 months).
Clinical presentation
The most common presenting symptom on hospital admission was headache in 20 (74.0%) patients. Visual field defects occurred in 8 (29.6%) patients. Nine patients had endocrine disorders, including hyperprolactinemia (five patients), menstrual irregularity (five patients), DI (two patients), panhypopituitarism (two patients), galactorrhea (one patient), hypocortisolemia (one patient), and libido changes (one patient). One of the patients was diagnosed incidentally following a diagnostic workup for another condition and after a comprehensive explanation about her situation, she decided to choose surgical treatment. Formal preoperative visual field perimetry testing demonstrated that 8 (29.6%) of the patients had visual field deficits. A summary of the clinical findings is presented in
Imaging
Typical appearance of symptomatic RCC is said to be most often iso- to hyperintense on T1-weighted image (WI) and hyperintense on T2WI, and isointense on CT scans. However, we observed various types of intensity. In six cases, the lesion was isointense on T2, and in one case, the tumor was hypointense on T2-weighted MRI. Enhancement of the cyst wall was not rare and was observed in 7 (25.9%) cases, whereas complete enhancement of the lesion occurred in 4 (14.8%) cases.
In 16 (59.2%) cases, the tumor was primarily located in the sella turcica, whereas in 2 (7.4%) cases, it was primarily suprasellar. Nine (33.3%) cases had a secondary suprasellar extension. One case had a very huge tumor at the time of diagnosis and showed an unusual extension to the parasellar region and the middle fossa [
Surgical treatment
RCC removal was performed through a standard EETS approach except for two cases [
Figure 2:
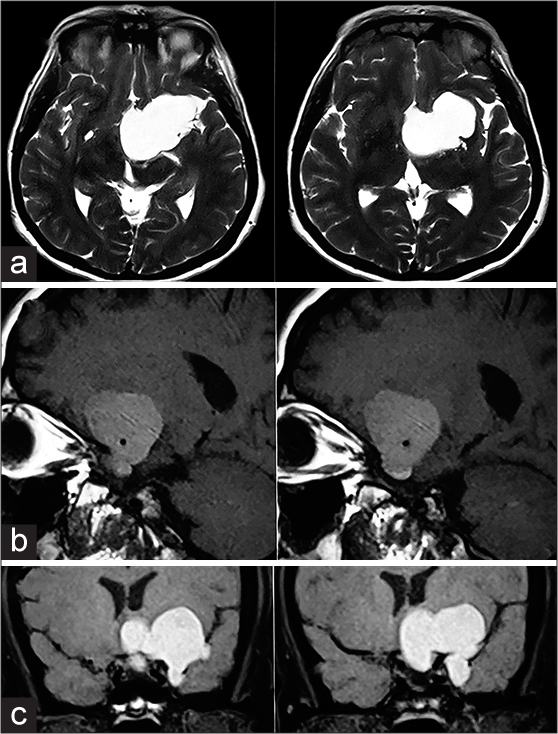
Sagittal (a) and axial (b) MRI images of a 42-year-old woman who presented with headache and showed a sellar lesion with ring enhancement. After 2 months, this patient refrained from surgery and returned with acute vision loss and severe headache. The new MRI (c and d) showed the lesion’s rapid progression, which caused a compressive effect on the optic structures. The patient was operated by extended EETS and almost all symptoms resolved after surgery.
One patient refused surgical treatment initially but returned after 2 months with exacerbation of his condition. He showed rapid growth of the lesion and presented with acute vision loss and visual field disturbance [
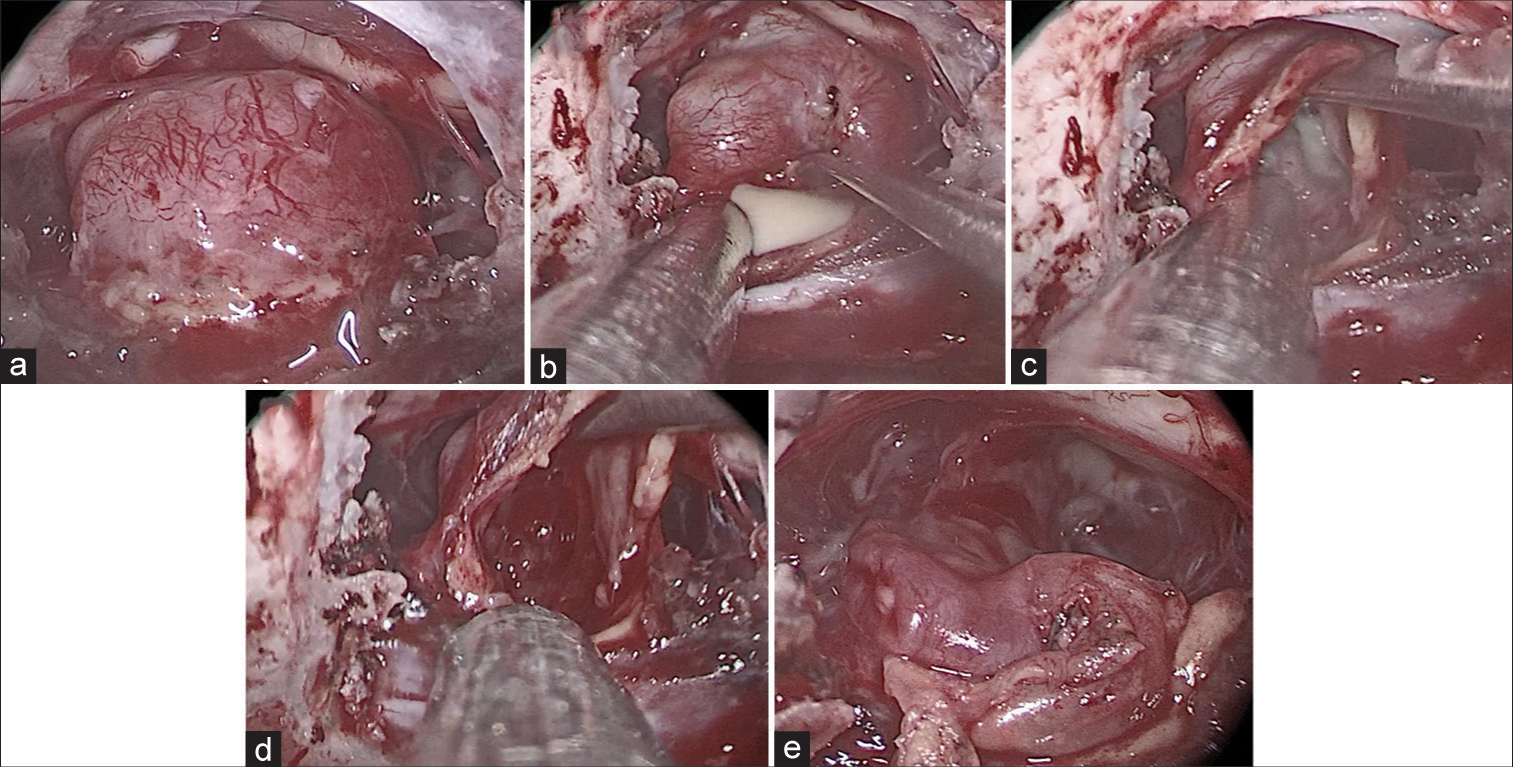
Figure 3:
(a) Endoscopic transsphenoidal view of a Rathke’s cleft cyst patient after drilling sellae and opening the dura. The pituitary gland surrounded the cyst circumferentially. Pituitary stalk and optic chiasma can be seen above the surgical field. (b) Trans-pituitary approach to Rathke’s cleft cyst. Colloid-like material drained after pituitary incision. (c) Cyst wall adhesion to the pituitary gland can be seen. (d) Removing cyst material and cyst wall as far as feasible. (e) Saving pituitary gland and stalk at the end of the operation.
Pathological analysis
The cyst walls and removed contents from all 27 patients were sent for analysis. Of the latter, 23 (85.1%) were identified as cuboidal or columnar epithelium. Four (14.8%) patients showed squamous metaplasia on histologic examination, among them two showed enhanced signal, while two were hypointense on T2-weighted MRI.
Surgical complications outcome
There was no major complication following surgery, except one patient who presented with acute visual deterioration 3 days after surgery. This event was due to chiasmal herniation into the sella, which resolved completely after chiasmopexy surgery. Transient DI happened in three cases which prolonged the duration of hospitalization to control hypernatremia; however, no permanent DI occurred following the surgery. Although we performed an extended endoscopic transplanum approach in ten cases to remove the suprasellar component of the lesion, we experienced no CSF leak following surgery.
Headaches resolved in 19 out of 20 patients who had it (95% recovery). Only one patient continued to have an intermittent low-intensity headache and was later treated medically. Seven (87.5%) out of eight patients with preoperative visual loss experienced an improvement in their vision following surgery. Six patients had complete improvement and one case had partial improvement. The preoperative visual acuity defect in one patient remained unchanged. Of those patients presenting with endocrinopathy, 55% had improvement of the anterior pituitary axis; hyperprolactinemia resolved in all patients; normalization of menstruation occurred in 3 (out of 5) women with oligomenorrhea. In the two patients presenting with DI, two with panhypopituitarism, and one with hypocortisolemia, persistent postoperative hormone replacement was commenced.
Follow-up
In a mean follow-up of 18 months, we observed no recurrence of RCC in our patients. Almost 95% of the cases had complete relief after surgery, and in one patient, the chronic persistent headache became an intermittent headache with the lower intensity in comparison with the preoperative condition. His headache responded to medication successfully.
DISCUSSION
RCC is a rare diagnosis in neurosurgery that can occur in every age group and it becomes symptomatic when it is large enough to compress the adjacent structures or rupture.[
Endocrine dysfunction has been described in 17–81% of patients with RCC, and hyperprolactinemia was the most common abnormality.[
Surgical intervention is the mainstay for the management of symptomatic RCC. The transsphenoidal approach has become the standard route for intrasellar lesion and EETS has proved its efficacy to standard approaches as reported in the previous studies.[
MRI is the ideal modality for preoperative assessment of RCCs and for distinguishing RCCs from other cystic sellar lesions. RCCs can be diagnosed on MRI based on shape, signal intensity, enhancement features, and an intracystic nodule. The diverse appearance of RCCs on MRI makes the neuroimaging diagnosis of an RCC difficult.[
Many other neoplastic and nonneoplastic suprasellar lesions can clinically and radiologically mimic the RCC, including pituitary adenomas, craniopharyngiomas, germ cell tumors, suprasellar meningiomas, midline gliomas, hypothalamic hamartomas, Langerhans cell histiocytosis, tuberculoma, and sarcoidosis.[
There is no general consensus regarding the extent of resection and debate still exists between radical cyst wall excision and partial cyst excision with cyst drainage. Xie et al. suggested that fenestration and aspiration of the cysts with partial excision of the cyst wall are usually sufficient.[
Recent case-series have reported an improvement in visual symptoms in 68–98% of the patients; however, total recovery and improvement were not differentiated.[
RCC may remain stable throughout the patient’s life. Conservative management and MRI follow-up are suitable for silent RCC or smaller cysts with mild symptoms, particularly in the elderly or younger patients without fertility.[
The recurrence rate in the literature varies from 0% to 40%.[
Study limitations
Our sample size and retrospective nature of the study are the major limiting factors of our work. Furthermore, this was a single-center experience and as we are one of the few centers for skull base surgery in Iran, it is probable that more complicated patients present to our center, and this may influence our findings. We also think that a longer follow-up period could help to achieve more clear-cut results.
CONCLUSION
Our experience with RCC patients showed that EETS is a safe and effective method in the treatment of patients with minimal recurrence.
Ethics approval
Ethics committee of Shahid Beheshti University of Medical Sciences.
Availability of data and material
Data are available.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005. 102: 189-93
2. Alfieri A, Schettino R, Tarfani A, Bonzi O, Rossi GA, Monolo L. Endoscopic endonasal removal of an intra-suprasellar Rathke’s cleft cyst: Case report and surgical considerations. Minim Invasive Neurosurg. 2002. 45: 47-51
3. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts: Technical considerations and outcomes. J Neurosurg. 2004. 101: 577-84
4. Billeci D, Marton E, Tripodi M, Orvieto E, Longatti P. Symptomatic Rathke’s cleft cysts: A radiological, surgical and pathological review. Pituitary. 2004. 7: 131-7
5. Binning MJ, Gottfried ON, Osborn AG, Couldwell WT. Rathke cleft cyst intracystic nodule: A characteristic magnetic resonance imaging finding. J Neurosurg. 2005. 103: 837-40
6. Bommakanti K, Panigrahi M, Yarlagadda R, Sundaram C, Uppin MS, Purohit AK. Optic chiasmatic-hypothalamic gliomas: Is tissue diagnosis essential?. Neurol India. 2010. 58: 833-40
7. Brassier G, Morandi X, Tayiar E, Riffaud L, Chabert E, Heresbach N. Rathke’s cleft cysts: Surgical-MRI correlation in 16 symptomatic cases. J Neuroradiol. 1999. 26: 162-71
8. Byun WM, Kim OL, Kim D. MR imaging findings of Rathke’s cleft cysts: Significance of intracystic nodules. AJNR Am J Neuroradiol. 2000. 21: 485-8
9. Cappabianca P, Cavallo LM, Colao A, de Divitiis E. Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg. 2002. 97: 293-8
10. Cavallo LM, Prevedello D, Esposito F, Laws ER, Dusick JR, Messina A. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008. 31: 55-64
11. Chaiban JT, Abdelmannan D, Cohen M, Selman WR, Arafah BM. Rathke cleft cyst apoplexy: A newly characterized distinct clinical entity. J Neurosurg. 2011. 114: 318-24
12. Chibbaro S, Signorelli F, Milani D, Cebula H, Scibilia A, Bozzi MT. Primary endoscopic endonasal management of giant pituitary adenomas: Outcome and pitfalls from a large prospective multicenter experience. Cancers (Basel). 2021. 13: 3603
13. Chotai S, Liu Y, Pan J, Qi S. Characteristics of Rathke’s cleft cyst based on cyst location with a primary focus on recurrence after resection. J Neurosurg. 2015. 122: 1380-9
14. Christophe C, Flamant-Durand J, Hanquinet S, Heinrichs C, Raftopoulos C, Sariban E. MRI in seven cases of Rathke’s cleft cyst in infants and children. Pediatr Radiol. 1993. 23: 79-82
15. El-Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic Rathke’s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998. 42: 7-16
16. Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: Ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011. 96: 1633-41
17. Fan J, Peng Y, Qi S, Zhang XA, Qiu B, Pan J. Individualized surgical strategies for Rathke cleft cyst based on cyst location. J Neurosurg. 2013. 119: 1437-46
18. Frank G, Sciarretta V, Mazzatenta D, Farneti G, Modugno GC, Pasquini E. Transsphenoidal endoscopic approach in the treatment of Rathke’s cleft cyst. Neurosurgery. 2005. 56: 124-8
19. Gaddikeri S, Vattoth S, Riley KO, DeHoff GW, Smith CB, Combs JT. Rathke cleft cyst, MRI criteria for presumptive diagnosis. Neurosciences (Riyadh). 2013. 18: 258-63
20. Hama S, Arita K, Nishisaka T, Fukuhara T, Tominaga A, Sugiyama K. Changes in the epithelium of Rathke cleft cyst associated with inflammation. J Neurosurg. 2002. 96: 209-16
21. Higgins DM, Van Gompel JJ, Nippoldt TB, Meyer FB. Symptomatic rathke cleft cysts: Extent of resection and surgical complications. Neurosurg Focus. 2011. 31: E2
22. Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic Rathke’s cleft cyst. Clin Neurol Neurosurg. 2001. 103: 96-100
23. Kanter AS, Sansur CA, Jane JA, Laws ER. Rathke’s cleft cysts. Front Horm Res. 2006. 34: 127-57
24. Kim CW, Hwang K, Joo JD, Kim YH, Han JH, Kim CY. Spontaneous involution of rathke’s cleft cysts without visual symptoms. Brain Tumor Res Treat. 2016. 4: 58-62
25. Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG. Surgical treatment of symptomatic Rathke cleft cysts: Clinical features and results with special attention to recurrence. J Neurosurg. 2004. 100: 33-40
26. Koutourousiou M, Grotenhuis A, Kontogeorgos G, Seretis A. Treatment of Rathke’s cleft cysts: Experience at a single centre. J Clin Neurosci. 2009. 16: 900-3
27. Kumar J, Kumar A, Sharma R, Vashisht S. Magnetic resonance imaging of sellar and suprasellar pathology: A pictorial review. Curr Probl Diagn Radiol. 2007. 36: 227-36
28. Larkin S, Karavitaki N, Ansorge O. Rathke’s cleft cyst. Handb Clin Neurol. 2014. 124: 255-69
29. Laws ER, Kanter AS. Rathke cleft cysts. J Neurosurg. 2004. 101: 571-2
30. Lemm D, de Oliveira FH, Bernays RL, Kockro RA, Kollias S, Fischer I. Rare suprasellar glioblastoma: Report of two cases and review of the literature. Brain Tumor Pathol. 2012. 29: 216-20
31. Mendelson ZS, Husain Q, Elmoursi S, Svider PF, Eloy JA, Liu JK. Rathke’s cleft cyst recurrence after transsphenoidal surgery: A meta-analysis of 1151 cases. J Clin Neurosci. 2014. 21: 378-85
32. Nishioka H, Haraoka J, Izawa H, Ikeda Y. Headaches associated with Rathke’s cleft cyst. Headache. 2006. 46: 1580-6
33. Niwa J, Tanabe S, Ibayashi Y, Hashi K. Clinicopathological findings in symptomatic Rathke’s cleft cyst: Correlation between enhancement effects on MRI and histopathology of the cyst wall. Neurol Surg. 1996. 24: 125-33
34. Park M, Lee SK, Choi J, Kim SH, Kim S, Shin NY. Differentiation between cystic pituitary adenomas and Rathke cleft cysts: A diagnostic model using MRI. Am J Neuroradiol. 2015. 36: 1866-73
35. Park M, Lee SK, Choi J, Kim SH, Kim SH, Shin NY. Differentiation between cystic pituitary adenomas and rathke cleft cysts: A diagnostic model using MRI. AJNR Am J Neuroradiol. 2015. 36: 1866-73
36. Peng Y, Fan J, Li Y, Qiu M, Qi S. The supraorbital keyhole approach to the suprasellar and supra-intrasellar rathke cleft cysts under pure endoscopic visualization. World Neurosurg. 2016. 92: 120-5
37. Rao VJ, James RA, Mitra D. Imaging characteristics of common suprasellar lesions with emphasis on MRI findings. Clin Radiol. 2008. 63: 939-47
38. Ratha V, Patil S, Karmarkar VS, Shah NJ, Deopujari CE. Surgical management of rathke cleft cysts. World Neurosurg. 2017. 107: 276-84
39. Ross DA, Norman D, Wilson CB. Radiologic characteristics and results of surgical management of Rathke’s cysts in 43 patients. Neurosurgery. 1992. 30: 173-8
40. Saeki N, Sunami K, Sugaya Y, Yamaura A. MRI findings and clinical manifestations in Rathke’s cleft cyst. Acta Neurochir (Wien). 1999. 141: 1055-61
41. Teramoto A, Hirakawa K, Sanno N, Osamura Y. Incidental pituitary lesions in 1,000 unselected autopsy specimens. Radiology. 1994. 193: 161-4
42. Tominaga JY, Higano S, Takahashi S. Characteristics of Rathke’s cleft cyst in MR imaging. Magn Reson Med Sci. 2005. 2: 1-8
43. Wait SD, Garrett MP, Little AS, Killory BD, White WL. Endocrinopathy, vision, headache, and recurrence after transsphenoidal surgery for Rathke cleft cysts. Neurosurgery. 2010. 67: 837-43
44. Wajima D, Yonezawa T, Masui K, Aketa S. Relationship between clinical features and T2-weighted magnetic resonance images in symptomatic rathke cleft cysts. World Neurosurg. 2016. 88: 421-7
45. Xie T, Hu F, Yu Y, Gu Y, Wang X, Zhang X. Endoscopic endonasal resection of symptomatic Rathke cleft cysts. J Clin Neurosci. 2011. 18: 760-2
46. Zada G, Lin N, Ojerholm E, Ramkissoon S, Laws ER. Craniopharyngioma and other cystic epithelial lesions of the sellar region: A review of clinical, imaging, and histopathological relationships. Neurosurg Focus. 2010. 28: E4
47. Zhong W, You C, Jiang S, Huang S, Chen H, Liu J. Symptomatic rathke cleft cyst. J Clin Neurosci. 2012. 19: 501-8