- Global Neurosurgical Alliance, Tucson, Arizona, United States
- College of Medicine, The University of Arizona College of Medicine - Tucson, Arizona, United States
- Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States
- College of Medicine, The University of Arizona College of Medicine - Phoenix, Arizona, United States,
- Faculty of Medicine, Islamic University of Gaza, Gaza, Palestine, Palestinian Territory, Occupied
- College of Medicine, Islamic University of Gaza, Rafah Refugee Camp, Rafah, Palestinian Territory, Occupied
- Department of Neurosurgery, Mayo Clinic, Rochester, Minnesota, United States
- Professor of Neurosurgery, Harvard Medical School, Brigham and Women’s Hospital, Boston, Massachusetts, United States.
Correspondence Address:
Albert Alan, Department of Neurosurgery, University of Arizona, Tucson, Arizona, United States.
DOI:10.25259/SNI_226_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Albert Alan1,2,3, Michelle Ennabe1,4, Muath Alsarafandi1,5,6, Noor Malik1,7, Edward R. Laws8, Martin Weinand2,3. Redefining cerebellar assessment: A comprehensive review of the cerebellum’s cognitive and affective roles and the efficacy of CCAS scales. 26-Apr-2024;15:141
How to cite this URL: Albert Alan1,2,3, Michelle Ennabe1,4, Muath Alsarafandi1,5,6, Noor Malik1,7, Edward R. Laws8, Martin Weinand2,3. Redefining cerebellar assessment: A comprehensive review of the cerebellum’s cognitive and affective roles and the efficacy of CCAS scales. 26-Apr-2024;15:141. Available from: https://surgicalneurologyint.com/surgicalint-articles/12876/
Abstract
Background: Emerging research expands our understanding of the cerebellum beyond motor control to include cognitive, emotional, and autonomic functions. This review examines the cerebellum’s complex role, spotlighting Schmahmann’s syndrome, or cerebellar cognitive affective syndrome (CCAS), which impairs executive functions, language, and spatial processing. It emphasizes advancements in diagnosing CCAS and the imperative of developing superior diagnostic tools for managing cerebellar pathologies effectively.
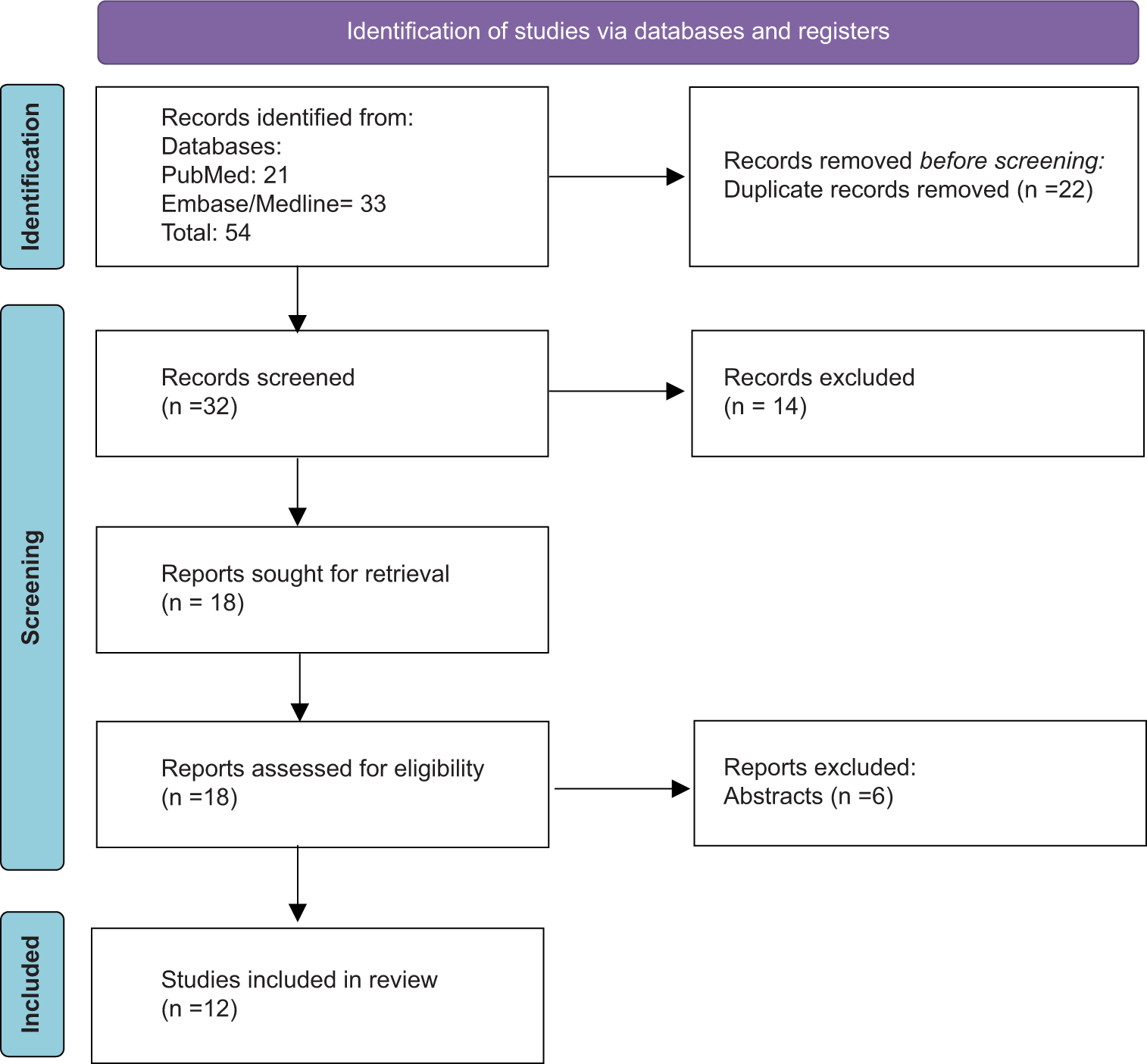
Methods: A comprehensive literature search was performed using databases such as PubMed, OVID Embase, and OVID Medline. Using the keywords “cerebellar cognitive, affective syndrome” and “Schmahmann syndrome,” the search was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines for systemic review, in which the selection process narrowed down an initial set of 54 articles to 12, focusing on the impact of the CCAS scale on diagnosing and understanding Schmahmann’s syndrome.
Results: The review’s analysis confirms the cerebellum’s roles in motor and cognitive functions and underscores the CCAS scale as a significant advancement in detecting cerebellar deficits, surpassing traditional assessments such as the mini-mental state examination and Montreal cognitive assessment.
Conclusion: This review emphasizes the importance of understanding the cerebellum’s involvement in cognition and emotion and the crucial role of the CCAS scale for identifying cerebellar impairments. It calls for better diagnostic tools to assess CCAS accurately and suggests enhancing the CCAS Scale to reflect cultural and educational diversity. This will improve the diagnosis and treatment of cerebellar disorders, promoting a comprehensive neurological perspective on the cerebellum’s functions.
Keywords: Cerebellar cognitive affective syndrome scale, Cerebellar cognitive affective syndrome (CCAS), Cerebellum, Mini-mental state examination (MMSE), Schmahmann’s syndrome
INTRODUCTION
For many years, the cerebellum’s significance was circumscribed to motor control, with historical literature predominantly focusing on its role in motor deficits.[
Contemporary research has significantly broadened this narrow view, uncovering the cerebellum’s integral functions across a spectrum of neurological domains – ranging from sensory to cognitive, emotional, social, psychological, and autonomic systems.[
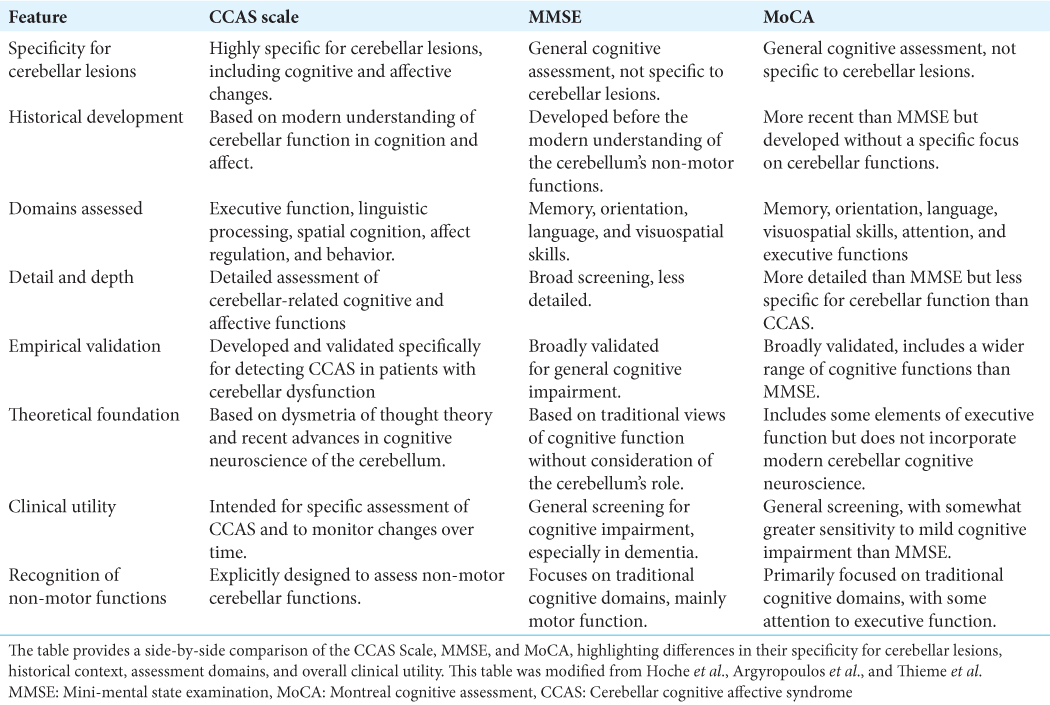
Despite the increasing recognition of Schmahmann’s syndrome, comprehensive insights into its mechanisms and effects are still lacking. This review seeks to consolidate our current understanding by drawing from an extensive peer-reviewed literature search that prioritizes studies offering substantial contributions to the multidimensional comprehension of CCAS. The examination presented delves into the diagnostic capabilities of scales such as the mini-mental state examination (MMSE), Montreal cognitive assessment (MoCA), and the more recent CCAS scale while also considering insights from case reports and comprehensive reviews.
This paper reviews a spectrum of studies, spanning from empirical research to case reports, to trace the evolving narrative of CCAS diagnoses. This examination not only illuminates the broader implications of the syndrome but also underscores the urgent need for refined diagnostic tools that keep pace with the expanding realm of cerebellar pathologies. By providing a comprehensive overview, this review aims to inform and potentially transform clinical practices, optimize the diagnosis and management of cerebellar cognitive and affective disorders, and foster a more profound appreciation of the cerebellum’s pivotal roles in the brain’s complex network.
METHODS
A comprehensive literature search was undertaken across PubMed and Embase/Medline databases to delve into the existing knowledge about the cerebellum’s influence on cognition and its relationship with Schmahmann syndrome. This exploration covered the history of each database up to November 2023.
The initial search strategy was confined to the terms “ cerebellar cognitive affective syndrome” and “Schmahmann syndrome” due to limited research on this condition. Articles were selected based on their exploration of the cerebellum’s broad influence in relation to Schmahmann syndrome.
Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines, the search strategy is detailed in
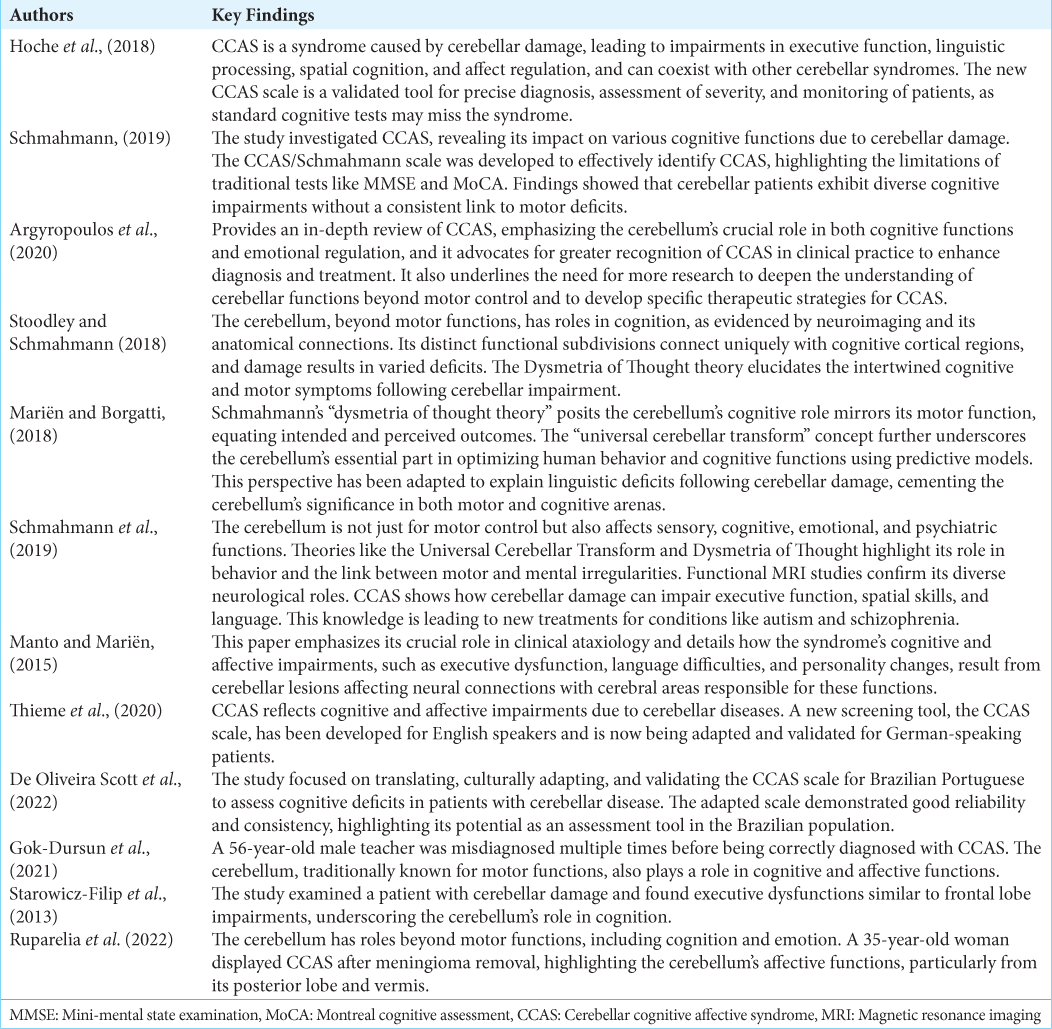
Of the 12 articles selected for detailed analysis, five provide comprehensive reviews of Schmahmann syndrome and reference a broader range of literature. Three of the articles are observational studies that contrast subjects from a typical control group with those diagnosed with CCAS. One article is a combined Literature Review and Clinical Observation. The remaining three articles are individual case reports, each emphasizing a direct correlation between cerebellar damage and CCAS.
FINDINGS FROM THE LITERATURE
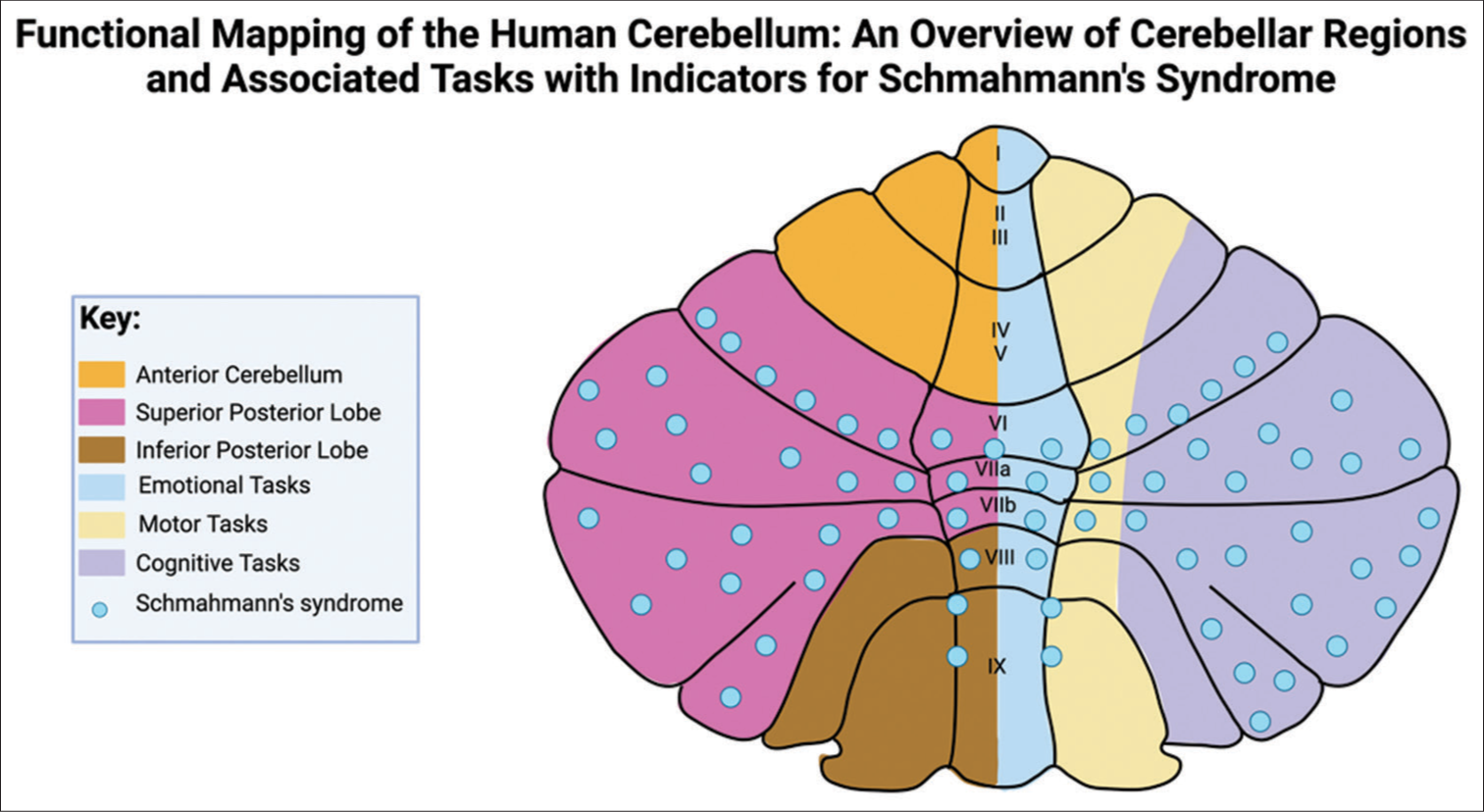
Figure 2:
Regional functional distribution in the cerebellum with Schmahmann’s syndrome indicators. This figure illustrates the cerebellum’s lobular division and associated functions, pinpointing regions implicated in Schmahmann’s syndrome. This figure was modified from Mariën and Borgatti and Manto and Mariën.
Expanding on the established research, Schmahmann et al. not only reinforce the cerebellum’s engagement in cognitive tasks but also introduce a theoretical model that underscores its precision in fine-tuning mental processes, akin to its role in motor coordination.[
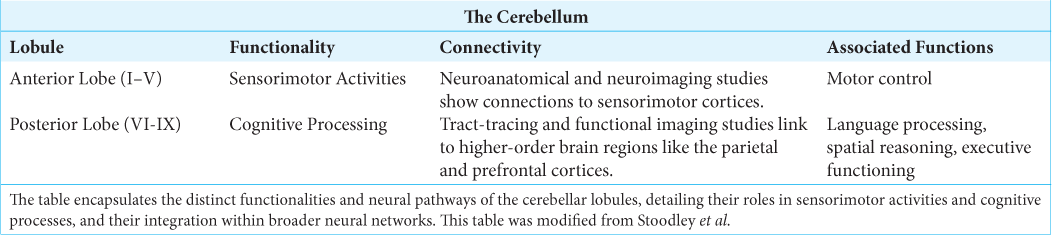
The insights from both Stoodley et al. and Schmahmann further consolidate the understanding that the cerebellum’s influence extends well beyond motor coordination, playing a substantial role in cognitive and emotional functioning. These findings articulate that while the anterior cerebellum primarily governs motor control, the posterior cerebellum is instrumental for cognitive and affective processing.[
A 2019 study by Argyropoulos et al. empirically validated the CCAS Scale, which highlights the extensive involvement of the cerebellum in cognitive and emotional functions, advocating for specialized assessments like the CCAS Scale in neurological evaluations.[
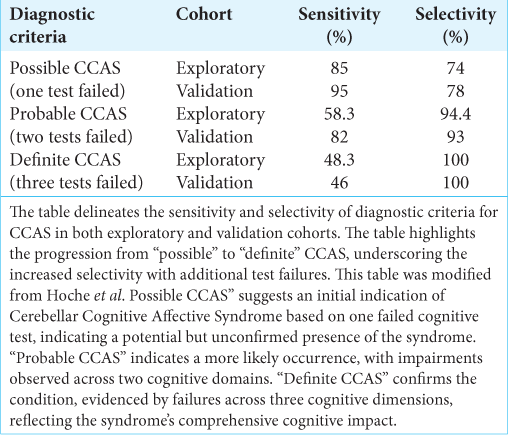
To address this diagnostic gap, the CCAS scale was developed, comprising various tests and measures that collectively provide a comprehensive assessment of cognitive and affective functions. This allows for a detailed understanding of the patient’s condition and aids in the differential diagnosis of CCAS from other neurological disorders. A study on the CCAS Scale involved two groups; the exploratory group was tested to refine the scale’s diagnostic criteria, while the validation group confirmed its efficacy. The “pass” system in the scale denotes the severity of CCAS: one failed test suggests possible CCAS, two failed tests indicate a probable condition, and three failed tests confirm definite CCAS. The exploratory cohort’s results highlighted the scale’s sensitivity in detecting early signs of CCAS despite a high false-positive rate. Conversely, the validation cohort’s results affirmed the scale’s improved sensitivity and robustness in confirming more severe cases. For instance,
Subsequent validation studies of the CCAS scale in Germany and Brazil have solidified its internal consistency and cultural adaptability, with Cronbach’s alpha scores well above the threshold of 0.7, which is considered acceptable for scales in psychological research; however, the scores were adjusted due to education. The German study yielded a score of 0.84, while the adaptation for Brazilian Portuguese achieved a score of 0.752, both indicative of good reliability.[
Illustrating the scale’s utility in a clinical context, a case report by Gok-Dursun et al. identifies a patient with CCAS initially misdiagnosed with psychiatric conditions, highlighting the scale’s practical use when standard neurological tests may miss subtle cognitive and affective symptoms post-cerebellar stroke.[
The analysis presented consolidates the understanding that the cerebellum plays a role in both motor and cognitive functions. The convergence of data from various studies underscores the necessity of incorporating specific assessment tools, such as the CCAS scale, into standard neurological practice to reflect the cerebellum’s broad contributions accurately. The empirical backing provided by the CCAS scale’s validation marks a critical step in the nuanced evaluation of cerebellar disorders, offering clinicians a more targeted approach to the diagnosis and treatment of cerebellar cognitive affective syndrome. This section has thus laid a robust foundation for the cerebellum’s multifaceted role in neurological function, with implications for future research and clinical application.
DISCUSSION
Recent neuroscientific research has expanded our understanding of the cerebellum beyond its classical role in sensorimotor control, as captured by the UCT model.[
The traditional view of the cerebellum as primarily a coordinator of motor function is challenged by the findings summarized in
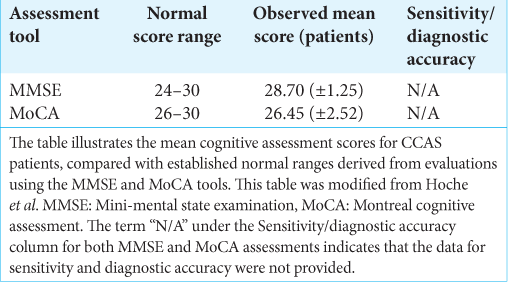
As such, the evolving comprehension of the cerebellum necessitates a reevaluation of the tools that we use for cognitive assessment, bridging us to the current standards of clinical practice. The MMSE and MoCA, while established as standard cognitive assessments, were not conceived with the nuanced roles of the cerebellum in cognition and affected in mind. This oversight often leads to the under-recognition of the cognitive and affective symptoms inherent to CCAS, which is evidenced by the analysis presented in
Recognizing these shortcomings highlights the need for more discerning evaluative measures, as further evidenced by the subsequent insights. The findings presented in
In light of this, the CCAS scale’s advancement in recognizing cerebellar cognitive-affective impairments is commendable, yet not without its own set of limitations.
This inconsistency hints at the potential for underdiagnosis of CCAS, particularly in its severe forms, which could result in patients being deprived of necessary specialized care. Conversely, the relatively high selectivity rates observed could also point to a potential overdiagnosis, driving home the necessity for a more nuanced diagnostic tool that prevents the misclassification of healthy individuals.
The cultural adaptation of the CCAS scale for the Brazilian Portuguese-speaking population underscores not just the inherent variability in diagnosing CCAS but also the critical necessity for sensitive calibration of the tool to align with an optimal balance of sensitivity and selectivity, aimed at approximately 70%.[
These diagnostic challenges are vividly illustrated in case reports from the literature, which depict the varied clinical presentations of CCAS and the conundrums faced in its diagnosis and management.[
Moreover, the necessity to adapt diagnostic tools like the CCAS scale to account for educational variations within patient populations further complicates the clinical picture. This complexity serves as a critical reminder of the educational factors that can significantly influence the interpretation of the CCAS scale, presenting a limitation in its applicability across different cultural and educational contexts. Ensuring that the CCAS Scale and similar tools are sufficiently adaptable for diverse patient populations is crucial, providing a reliable framework for evidence-based treatment and fostering better communication among physicians and specialists aware of this clinical entity.
Given the identified limitations of current assessment tools and the cultural and educational complexities highlighted in our findings, it becomes imperative for future research to concentrate on refining the CCAS scale to enhance its diagnostic precision across diverse populations. The necessity of integrating advanced neuroimaging modalities, such as Diffusion Tensor Imaging, directly responds to the need for a more granular view of the cerebellum’s connectivity, particularly in light of the variability in CCAS presentations and the overlap of symptoms with other neurological conditions.[
Moreover, an identifiable limitation arises from the limited volume of research specifically addressing Schmahmann’s syndrome, particularly from a neurosurgical standpoint. This scarcity is significant, as it suggests that the symptoms and signs of Schmahmann’s syndrome might be obscured by the broader spectrum of cerebellar surgery outcomes, potentially leading to an underdiagnosis or misinterpretation of its impact. The need for targeted neurosurgical research to distinguish the specific contributions of surgical interventions to the clinical picture of CCAS is urgent, highlighting a crucial area for future investigation.
Furthermore, the discovery and validation of biomarkers for neurotransmission, neuroinflammation, and genetic predispositions stand as a frontier to be explored, driven by the need for more nuanced diagnostic criteria that can differentiate CCAS from psychiatric and other neurological disorders. The establishment of these biomarkers could represent an advancement in diagnostic capabilities, providing a robust foundation for evidence-based practice. Complementing these scientific endeavors, the exploration of the cerebellum’s interactions with the broader cognitive network offers a potential revolution in cognitive rehabilitation techniques. By leveraging the brain’s neuroplasticity, informed by the educational implications discussed earlier, these innovative approaches could be tailored to accommodate the unique educational backgrounds and learning needs of patients with CCAS.
Ultimately, these concerted research efforts aim to fortify the CCAS scale’s applicability, ensuring it not only captures the nuances of CCAS presentations but also contributes meaningfully to patient management and treatment outcomes. Through such advancements, we endeavor to bridge the gaps in knowledge and clinical practice, thus enhancing the quality of life for those affected by this syndrome and expanding our comprehension of the cerebellum within the brain’s complex systems.
In conclusion, the findings and discussions articulated in this review underscore the need for a paradigm shift in how we perceive and assess the cerebellum’s role in cognitive and affective functions. The journey through extensive literature, nuanced case studies, and empirical validations leads us to a juncture where we must embrace the cerebellum’s complex contributions with renewed vigor. By advancing our research methodologies and refining our diagnostic tools, we are on the path to a more comprehensive understanding of cerebellar cognitive affective syndrome. The insights provided here advocate for continued innovation in clinical practice and research, setting the stage for enhanced patient care and a deeper grasp of the cerebellum’s multifaceted influence on neurological health.
CONCLUSION
This review asserts the cerebellum’s role in cognition and emotion, challenging its traditional motor-centric classification. This paper highlights the CCAS Scale’s superiority over conventional tools, such as the MMSE and MoCA, for diagnosing cerebellar cognitive affective syndrome, advocating for its refined use in clinical settings. Despite its efficacy, the scale’s sensitivity to cultural and educational variations calls for further refinement. Future work should aim to perfect this scale, explore novel biomarkers, and leverage advanced imaging to understand cerebellar dysfunction better. Ultimately, our findings call for a revised neurological paradigm that recognizes the cerebellum’s broader influence, paving the way for enhanced patient care and a comprehensive understanding of brain function.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Argyropoulos GP, van Dun K, Adamaszek M, Leggio M, Manto M, Masciullo M. The cerebellar cognitive affective/Schmahmann syndrome: A task force paper. Cerebellum. 2020. 19: 102-25
2. De Oliveira Scott SS, Pedroso JL, Elias VV, Nóbrega PR, Sobreira ES, de Almeida MP. Translation, cross-cultural adaptation, and validation to brazilian portuguese of the cerebellar cognitive affective/Schmahmann syndrome scale. Cerebellum. 2022. 22: 282-94
3. Gok-Dursun E, Gultekin-Zaim OB, Tan E, Unal-Cevik I. Cognitive impairment and affective disorder: A rare presentation of cerebellar stroke. Clin Neurol Neurosurg. 2021. 206: 106690
4. Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018. 141: 248-70
5. Manto M, Mariën P. Schmahmann’s syndrome-identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias. 2015. 2: 2
6. Mariën P, Borgatti R. Language and the cerebellum. Handb Clin Neurol. 2018. 154: 181-202
7. Ruparelia J, Gosal JS, Kokkula P, Garg M, Bhaskar S, Panda S. The Yin and Yang of operating on a posterior fossa meningioma: The Schmahmann syndrome. Neurol India. 2022. 70: 1661-64
8. Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019. 42: 337-64
9. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. 2019. 688: 62-75
10. Starowicz-Filip A, Milczarek O, Kwiatkowski S, BętkowskaKorpała B, Prochwicz KA. Cerebellar cognitive affective syndrome CCAS--a case report. Arch Psychiatry Psychother. 2013. 15: 57-64
11. Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol. 2018. 154: 59-70
12. Thieme A, Roeske S, Faber J, Sulzer P, Minnerop M, Elben S. Validation of a German version of the cerebellar cognitive affective/Schmahmann syndrome scale: Preliminary version and study protocol. Neurol Res Pract. 2020. 2: 39