- Instituto do Cancer do Estado de Sao Paulo, Hospital das Clinicas, Faculdade de Medicina da Universidade de São Paulo,
- Department of Neurology, Faculdade de Medicina da Universidade de São Paulo,
- Department of Neurosurgery, Eberhard Karls University, Tübingen, Germany.
Correspondence Address:
Alexandra Gomes dos Santos
Department of Neurology, Faculdade de Medicina da Universidade de São Paulo.
DOI:10.25259/SNI_538_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Iuri Santana Neville1, Alexandra Gomes dos Santos2, Cesar Cimonari Almeida2, Leonardo Bilich Abaurre2, Samia Yasin Wayhs2, Olavo Feher1, Manoel Jacobsen Teixeira2, Guilherme Lepski2,3. Reoperation for recurrent glioblastomas: What to expect?. 03-Feb-2021;12:42
How to cite this URL: Iuri Santana Neville1, Alexandra Gomes dos Santos2, Cesar Cimonari Almeida2, Leonardo Bilich Abaurre2, Samia Yasin Wayhs2, Olavo Feher1, Manoel Jacobsen Teixeira2, Guilherme Lepski2,3. Reoperation for recurrent glioblastomas: What to expect?. 03-Feb-2021;12:42. Available from: https://surgicalneurologyint.com/surgicalint-articles/reoperation-for-recurrent-glioblastomas-what-to-expect/
Abstract
Background: The current standard treatment for glioblastoma (GBM) is maximal safe surgical resection followed by radiation and chemotherapy. Unfortunately, the disease will invariably recur even with the best treatment. Although the literature suggests some advantages in reoperating patients harboring GBM, controversy remains. Here, we asked whether reoperation is an efficacious treatment strategy for GBM, and under which circumstances, it confers a better prognosis.
Methods: We retrospectively reviewed 286 consecutive cases of newly diagnosed GBM in a single university hospital from 2008 to 2015. We evaluated clinical and epidemiological parameters possibly influencing overall survival (OS) by multivariate Cox regression analysis. OS was calculated using the Kaplan–Meier method in patients submitted to one or two surgical procedures. Finally, the survival curves were fitted with the Weibull model, and survival rates at 6, 12, and 24 months were estimated.
Results: The reoperated group survived significantly longer (n = 63, OS = 20.0 ± 2.3 vs. 11.4 ± 1.0 months, P P
Conclusion: Our data support the indication of reoperation for GBM, especially for younger patients with good functional status. Under these circumstances, survival can be doubled at 12 and 24 months.
Keywords: Brain tumor, Glioblastoma, Reoperation
INTRODUCTION
Glioblastomas (GBMs) account for 14.6% of all primary central nervous system tumors and 48.3% of all malignant tumors. The incidence of GBM is greater in males (1.58 M:1.0 F) and Caucasians (1.95 times greater than in Afro-descendants) and increases with age (median age at diagnosis = 65). Standard of care for patients younger than 70 years old and with a Karnofsky Performance Status (KPS) > 70 is surgical resection, adjuvant radiotherapy plus temozolomide, followed by six cycles of temozolomide, which confers a median progression-free survival (PFS) of 4.4–8.4 months.[
Almost inevitably recurrent, the rate of survival after 1 year of diagnosis is 40.8%, declining to 18.5% after 2 years and to 6.8% in 5 years, with a median overall survival (OS) of 12–15 months.[
Reoperation for recurrent GBM is indicated for 3–30% of cases and remains controversial due to the lack of high-level evidence (randomized double-blind trials). Furthermore, the survival benefit reported by some case series may be associated with confounding factors, such as adjuvant therapies, patients’ performance status, tumor location and volume, and genetic profile.[
Here, we asked whether reoperation is an efficacious treatment strategy for GBM, and under which circumstances, it should be indicated.
MATERIALS AND METHODS
Case series
This retrospective study reviewed 286 consecutive cases of newly diagnosed GBM at a single institution (Instituto do Cancer do Estado de São Paulo – Universidade de São Paulo), from 2008 to 2015. All patients were between 18 years old and 82 years old. Patients were divided into two groups: the one surgery group, including patients who underwent surgical resection once during GBM treatment, and the reoperation group, including patients who were reoperated during follow-up, undergoing two or more interventions. Patients submitted to stereotactic biopsy, for whom surgical resection was judged risky or not indicated, were excluded from the comparative survival analyses.
Management
Brain tumor diagnosis was performed based on clinical history, preoperative magnetic resonance imaging (MRI), and histopathologic study, as per the latest World Health Organization Classification of Tumors of the Central Nervous System.[
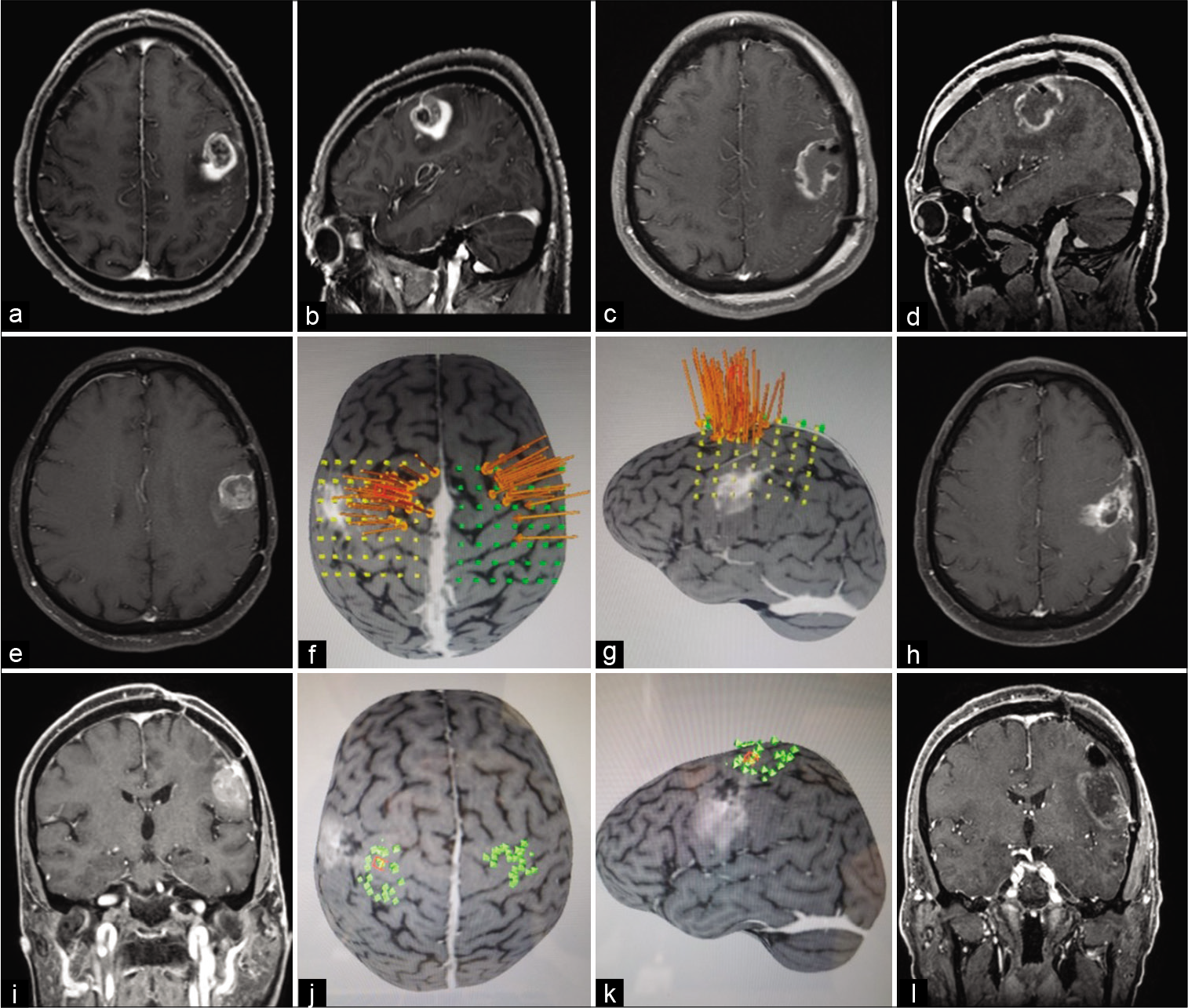
Figure 1:
This patient presented a wild-type isocitrate dehydrogenase glioblastoma on the left frontal lobe and underwent three resections. Preoperative postgadolinium T1 magnetic resonance imaging (MRI) axial (a), sagittal (b), early postoperative MRI axial (c), sagittal (d), second resection preoperative postgadolinium T1 MRI axial (e), preoperative motor cortex mapping using navigated transcranial magnetic stimulation (TMS): long arrows point location of M1 activation using nTMS (f and g), late postoperative axial (h), third resection preoperative postgadolinium T1 MRI coronal (i), preoperative motor cortex mapping using navigated TMS (j and k), early postoperative coronal (l).
Statistical analyses
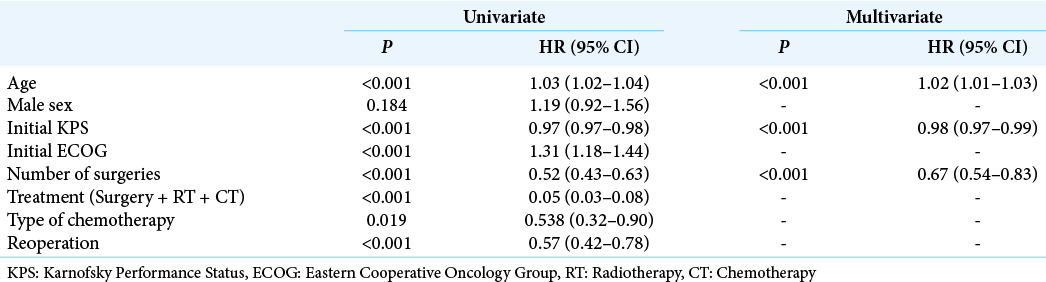
For statistical computations, we used JMP 14.2 (SAS Institute, CA, USA). We evaluated clinical and epidemiological parameters possibly influencing OS by multivariate analysis, REML estimation method (restricted maximal likelihood). The variables included were age, gender, initial KPS, number of surgeries, treatment strategy, and chemotherapy regimen. Next, OS from the time of diagnosis was calculated using the Kaplan–Meier method in patients submitted to one or two or more surgical procedures. Finally, the survival curves were fitted with the Weibull model, and survival probabilities at 6, 12, and 24 months were estimated. Significance was set at P < 0.01.
RESULTS
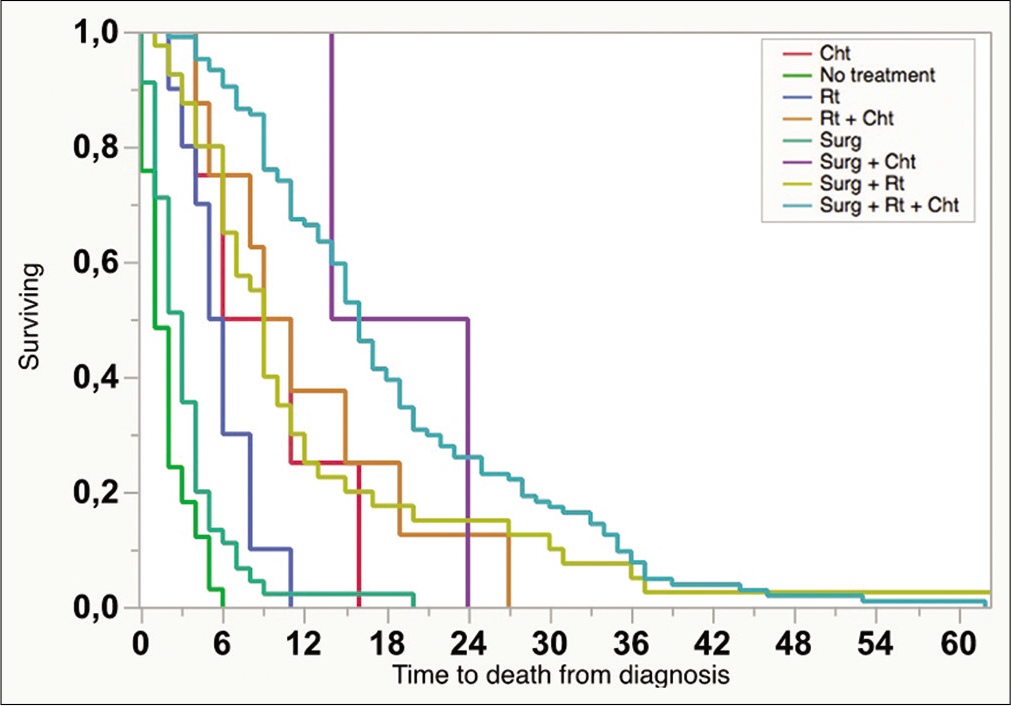
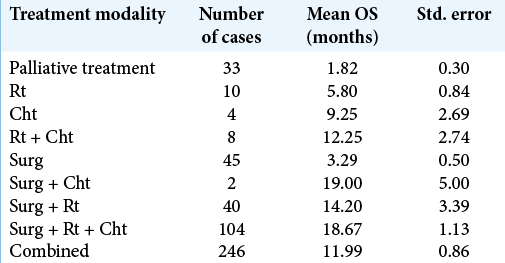
Patients’ mean age was 56.2 ± 13.9, and 60.5% were male. The mean preoperative KPS and ECOG scores were 73.5 ± 20.1 and 1.6 ± 1.4, respectively. Mean OS for the whole series (286 cases) was 8.5 (3–16) months. One hundred and twenty-three patients (43.0%) received standard of care treatment, composed of surgery, radiation, and chemotherapy. Fifty-three patients (18.5%) were submitted only to surgery, while 11 patients (3.8%) received radiotherapy and chemotherapy, and 4 patients (1.4%) only chemotherapy. The median time from surgery to radiation therapy was 70 days (45–104 days). Carmustine was the chemotherapy of choice for patients who were recruited between 2008 and 2010, while patients recruited later (84.6%) received temozolomide as the first-line drug. In line with previous findings, temozolomide was associated with longer OS compared with carmustine (19.36 ± 11.74 months vs. 12.95 ± 7.51, respectively, P < 0.05, Chi-square). Thirty-three patients (11.5%) received second-line chemotherapy. The OS survival of patients submitted to surgical resection was 14.2 ± 1.0 months.
Next, we analyzed 190 patients submitted to surgical resection (excluding those submitted to stereotactic biopsies and those who were not operated on). Patients were divided into two groups: those who underwent one single surgery and those who underwent two or more surgeries (one surgery group vs. reoperation group, respectively). The median time from the index surgery to the second surgery was 156 days (77–337 days). Patients in the reoperation group were slightly younger than patients in the one surgery group (50.5 ± 13.2 vs. 57.9 ± 12.8, respectively, P < 0.001), groups did not differ in terms of gender composition, and patients in the reoperation group presented slightly higher KPS compared with the one surgery group (82.2 ± 14.8 vs. 75.2 ± 18.8, P = 0.008,
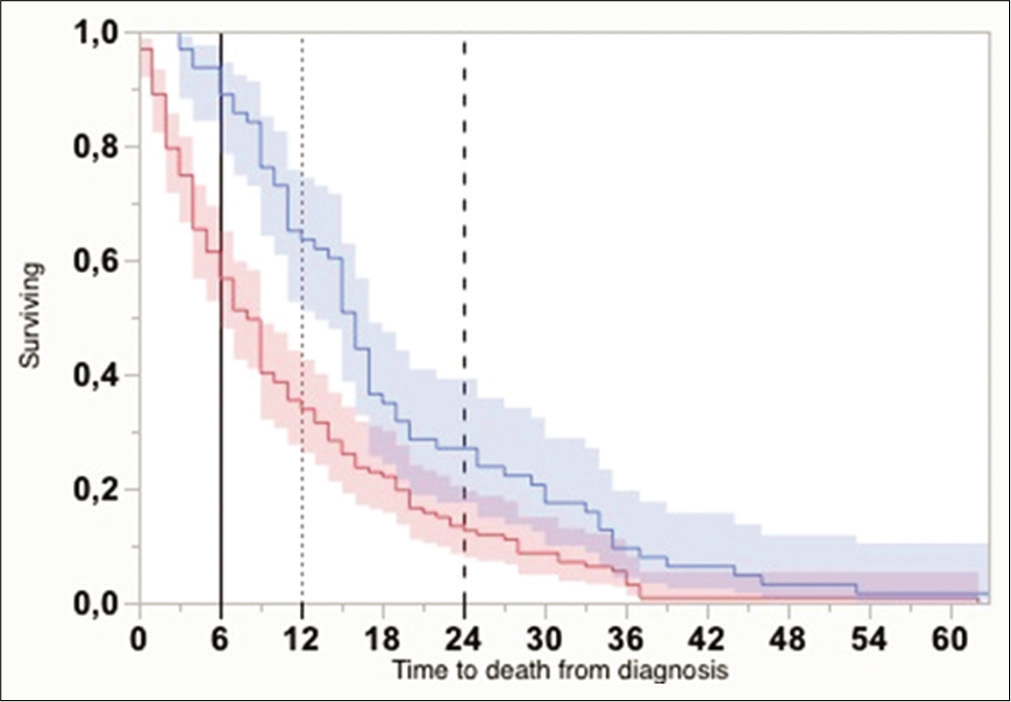
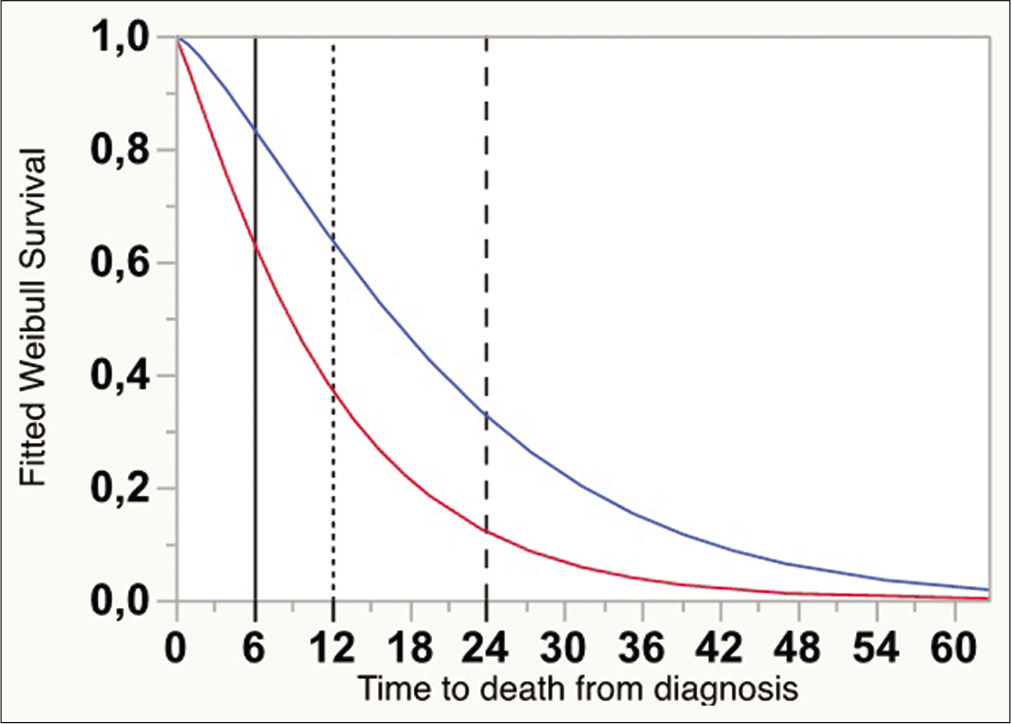
The Kaplan–Meier curves for OS (from the time of diagnosis) differed consistently between groups (one surgery: n = 127, OS = 11.4 ± 1.0 months vs. reoperation: n = 63, OS = 20.0 ± 2.3 months, Wilcoxon W = 21,30, P < 0.0001) [
Figure 2:
Kaplan–Meier curves with 95% confidential interval showing overall survival from the time of diagnosis, for cases submitted to one surgical intervention (darker gray), and two or more (lighter gray) (P < 0.0001, Wilcoxon). Vertical lines indicate the absolute times for survival estimate calculations (6, 12, and 24 months).
A mathematical regression for survival estimates was performed according to Weibull, which revealed that OS at 6, 12, and 24 months was significantly greater for the reoperation group [
DISCUSSION
Despite recent advances in the genetic profile of GBMs, an improved classification system, better neuroimaging techniques (which allow the early recognition of recurrence and the differential diagnosis of pseudoprogression and radionecrosis), and new technologies available in the operation theater (including better intraoperative mapping techniques, intraoperative imaging, and better neuroanesthesiological protocols), these patients continue to face very disappointing long-term survival rates. In fact, disease progression is virtually inevitable and occurs at a median of 6.9 months.[
Based on the paucity of efficacious alternatives, reoperation is often considered. In a recent review about the effectiveness of reoperation in GBM in the post temozolomide era, Robin et al. observed a median OS of 9.9 months after reintervention. Some predictors of improved survival after reoperation were as follows: (i) performance status, (ii) age, (iii) focal versus multifocal disease, (iv) favorable disease location, (v) lower preoperative tumor size, and (vi) greater likelihood of complete resection and safe surgery.[
In favor of reoperation, van Linde et al. performed a multicenter retrospective analysis comparing four groups of patients with recurrent GBMs according to treatment: systemic treatment (SYST), reresection followed by systemic treatment and/or reirradiation (SURG), reirradiation (RT), and best supportive care. After post hoc corrections, the SYST, SURG, and RT subgroups did not differ significantly in terms of age, gender, time of recurrence, performance status (KPS and ECOG), or OS. However, patients in the SURG group had greater PFS compared with patients in the SYST group, even after correcting for potential confounders. No differences were found between the SURG and RT groups.[
In contrast, Sastry et al. observed that reresection at the time of recurrence was not significantly associated with increased postprogression survival (PPS), which was only influenced by three variables: good performance status (KPS > 70), treatment with bevacizumab, and cytotoxic chemotherapy (all at first progression).[
It is important to note that in many studies, patients who underwent a new surgical procedure were usually younger (<65), had a better performance status (KPS > 80, ECOG < 2), and a lower degree of necrosis and enhancement on preoperative MRI, factors that are independently associated with increased survival.[
Another confounding factor in some retrospective analyses is that the nonoperated group usually includes a subgroup of patients whose clinical profile would never render them potential candidates for surgery. Tully et al. addressed this potential bias by analyzing two cohorts of patients with recurrent GBM. At first glance, the reoperation group seemed to have better OS than the nonoperated group. However, this effect vanished after excluding a subgroup of patients with infest prognostic factors (aged over 70 years, posterior fossa location, multicentric manifestation, ECOG >2).[
Robin et al. previously reported some potential bias associated with those positive results.[
This retrospective case series has some limitations. First, patients submitted to reoperation were mostly younger with good performance status, highlighting a potential selection bias. Second, at the time data were collected, IDH1 and MGMT promoter methylation status were not available at our institution (as the largest public University Hospital in Brazil, budget limitations have always been a major concern). However, the effect of this limitation on external validation is small, as only 5–12% of GBMs have IDH-1 mutations.[
The decision to reoperate a patient with recurrent GBM is often made on an individual basis, taking into account the criteria listed above, as well as the benefits of potentially achieving gross total resection, alleviation of mass effect, the possibility of further biomolecular analysis, and enrollment in ongoing clinical trials. The results presented here clearly support reintervention, especially for younger patients with a good functional status. Thus, strict and regular clinical and radiological follow-up is critical for detecting radiological recurrence before any relevant clinical deterioration is observed.
CONCLUSION
Reoperation for recurrent glioblastoma is an option to be considered when the functional status is not compromised, and our data indicate that this subgroup of patients may profit in terms of overall survival.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013. 31: 3212-8
2. Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet. 1995. 345: 1008-12
3. Fatehi M, Hunt C, Ma R, Toyota BD. Persistent disparities in survival for patients with glioblastoma. World Neurosurg. 2018. 120: e511-6
4. Gately L, Collins A, Murphy M, Dowling A. Age alone is not a predictor for survival in glioblastoma. J Neurooncol. 2016. 129: 479-85
5. Hata N, Mizoguchi M, Kuga D, Hatae R, Akagi Y, Sangatsuda Y. First-line bevacizumab contributes to survival improvement in glioblastoma patients complementary to temozolomide. J Neurooncol. 2020. 146: 451-8
6. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005. 352: 997-1003
7. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001. 95: 190-8
8. Louis DN, Perry A, Reifenberger G, von Deimling A, FigarellaBranger D, Cavenee WK. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016. 131: 803-20
9. Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, Mampre D, Jackson C, Peterson J. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J Neurooncol. 2020. 147: 297-307
10. Mukherjee S, Wood J, Liaquat I, Stapleton SR, Martin AJ. Craniotomy for recurrent glioblastoma: Is it justified? A comparative cohort study with outcomes over 10 years. Clin Neurol Neurosurg. 2020. 188: 105568
11. .editors. NCCN: National Comprehensive Cancer Network Guidelines Version 2. 2020. Central Nervous System Cancers. 2020. p.
12. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019. 21: v1-v100
13. Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008. 321: 1807-12
14. Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol. 2010. 28: 2051-7
15. Robin AM, Lee I, Kalkanis SN. Reoperation for recurrent glioblastoma multiforme. Neurosurg Clin N Am. 2017. 28: 407-28
16. Ryu S, Buatti JM, Morris A, Kalkanis SN, Ryken TC, Olson JJ. The role of radiotherapy in the management of progressive glioblastoma: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014. 118: 489-99
17. Sastry RA, Shankar GM, Gerstner ER, Curry WT. The impact of surgery on survival after progression of glioblastoma: A retrospective cohort analysis of a contemporary patient population. J Clin Neurosci. 2018. 53: 41-7
18. Skardelly M, Dangel E, Gohde J, Noell S, Behling F, Lepski G. Prolonged temozolomide maintenance therapy in newly diagnosed glioblastoma. Oncologist. 2017. 22: 570-5
19. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352: 987-96
20. Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016. 18: 549-56
21. Sung KS, Roh TH, Moon JH, Kim EH, Kang SG, Kim SH. Treatment results for recurrent glioblastoma and alteration of programmed death-ligand 1 expression after recurrence. World Neurosurg. 2020. 135: e459-67
22. Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014. 15: 943-53
23. Tully PA, Gogos AJ, Love C, Liew D, Drummond KJ, Morokoff AP. Reoperation for recurrent glioblastoma and its association with survival benefit. Neurosurgery. 2016. 79: 678-89
24. Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci. 2018. 54: 7-13
25. Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: A randomized double-blind study. Neurosurgery. 1997. 41: 44-8
26. van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AM, Vandertop WP. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J Neurooncol. 2017. 135: 183-92
27. Weller M, van den Bent M, Tonn JC, Stupp R, Preusser M, Cohen-Jonathan-Moyal E. European association for neuro-oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017. 18: e315-29
28. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017. 377: 1954-63
29. Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009. 360: 765-73
30. Zhao YH, Wang ZF, Pan ZY, Péus D, Delgado-Fernandez J, Pallud J. A meta-analysis of survival outcomes following reoperation in recurrent glioblastoma: Time to consider the timing of reoperation. Front Neurol. 2019. 10: 286