- Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center, Houston, United States.
- Department of Neurological Surgery, UT Southwestern, Dallas, United States.
- Department of Neurosurgery, Baylor College of Medicine, Houston, Texas, United States.
Correspondence Address:
Matthew Muir, Department of Neurosurgery, The University of Texas M.D. Anderson Cancer Center, Houston, Texas, United States.
DOI:10.25259/SNI_418_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Matthew Muir1, Jeffrey I. Traylor2, Ron Gadot3, Rajan Patel3, Sujit S. Prabhu1. Repeat laser interstitial thermal therapy for recurrent primary and metastatic intracranial tumors. 22-Jul-2022;13:311
How to cite this URL: Matthew Muir1, Jeffrey I. Traylor2, Ron Gadot3, Rajan Patel3, Sujit S. Prabhu1. Repeat laser interstitial thermal therapy for recurrent primary and metastatic intracranial tumors. 22-Jul-2022;13:311. Available from: https://surgicalneurologyint.com/surgicalint-articles/11739/
Abstract
Background: Repeat craniotomy in patients with primary and metastatic brain tumors carries significant morbidity and can delay adjuvant treatments. Repeat laser interstitial thermal therapy (LITT) for recurrent disease has been described and could benefit patients with limited cytoreductive options. We aim to describe the indications, safety, and efficacy of repeat LITT for recurrent primary and metastatic intracranial tumors.
Methods: Patients undergoing repeat ablations for the same lesion were included in the study. We retrospectively analyzed 13 patients treated with 29 total LITT ablations.
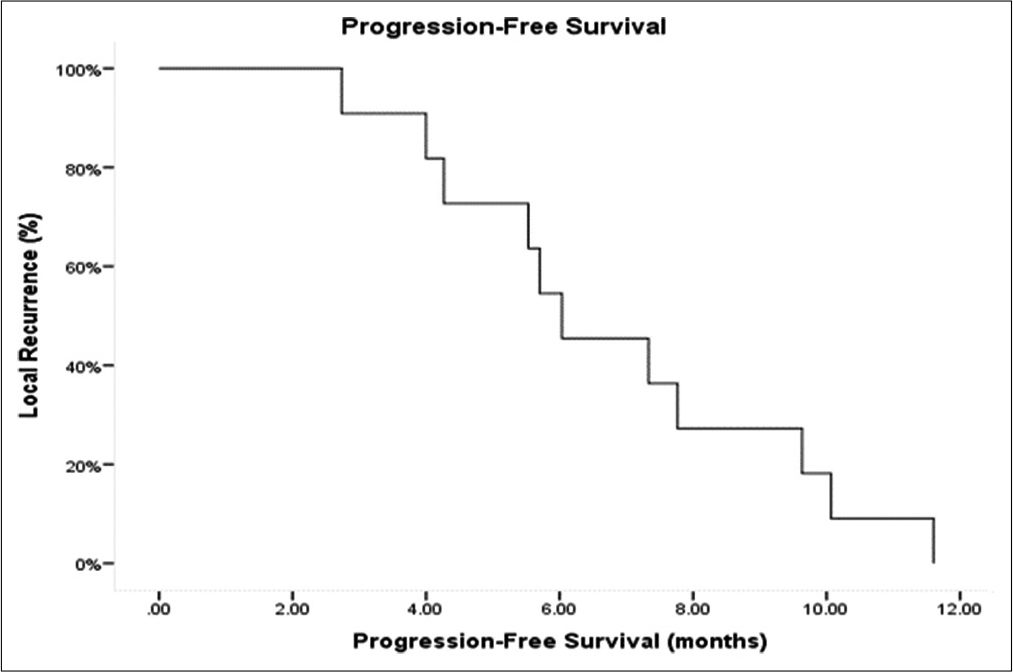
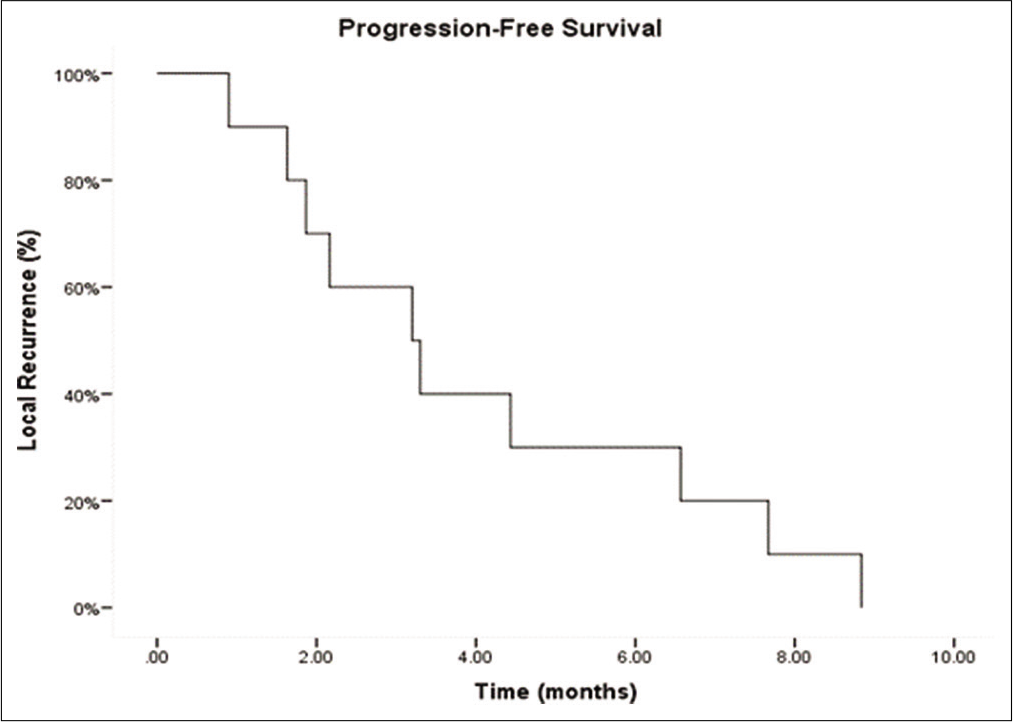
Results: Eleven patients were treated for glioblastoma (GBM), while two had brain metastases. Eleven patients had LITT performed only 2 times, while three patients underwent three total iterations of LITT for disease recurrence. Median length of stay after the 1st ablation was 2 days, while the median length of stay after the 2nd ablation was 1 day. The median time to resuming adjuvant treatments after the 1st LITT was 11 days. The median time to resuming adjuvant treatments after the 2nd LITT was 28 days. Four patients after the 1st and 2nd LITT sustained deficits persisting through 30-day follow-up. The median progression-free survival among the GBM patients from the first ablation was 6.0 months, 3.2 months from the 2nd ablation, and 2.1 months from the 3rd ablation.
Conclusion: Recurrent tumors, especially GBM, can be safely treated using repeat LITT when surgery cannot be effectively performed. Our results indicate that patients tolerate the procedure well and have a meaningful survival given the salvage nature of the procedure.
Keywords: Brain metastases, Glioblastoma, Laser interstitial thermal therapy
INTRODUCTION
Laser interstitial thermal therapy (LITT) has emerged as a novel minimally invasive, cytoreductive technique that has been used for the treatment of newly diagnosed and recurrent gliomas, epileptic foci, as well as recurrent brain metastases and radiation necrosis.[
Patients with glioblastoma (GBM) on an average have 2–3 craniotomies performed over the lifetime of the disease. Each intervention carries significant morbidity and delays in restarting adjuvant treatments. Patients with recurrent lesions following LITT have few options. These tumors present a unique challenge; the location of the tumor or the comorbidities and poor prognosis of the patients usually exclude the possibility of re-resection.[
MATERIALS AND METHODS
Study design, setting, and participants
This study was performed under the auspices of an Institutional Review Board-approved protocol in compliance with institutional regulations on the study of human subjects. A retrospective search of the departmental database at our institution from January 2013 to January 2018 identified 13 patients who treated with repeat LITT for the same lesion. Characteristics for pre- and post-LITT outcomes are reported.
Operative technique
All procedures were performed in an intraoperative magnetic resonance imaging (MRI) suite with a Siemens Espree 1.5 T magnet (Siemens, Berlin, Germany). A preoperative MRI was obtained to plan the trajectory of the ablation probe using Brainlab iPlan software (Brainlab, Munich, Germany). A cannulated bolt was then inserted in the patient’s skull under imaging guidance along the planned trajectory using the VarioGuide system (Brainlab) followed by insertion of the premeasured LITT1 probe. LITT procedures were performed by the senior authors with the NeuroBlate System (Monteris, Winnipeg, Canada) or the Visualase system (Medtronic, Minneapolis, Minnesota, USA). The NeuroBlate system was used on 11 patients, while the Visualase system was used on four patients. For two patients, a different system was used on each ablation. The details of our ablation technique have been described elsewhere.[
Follow-up, MRI, and volumetric analysis
All patients underwent brain MRI before the LITT procedure and follow-up imaging at regular intervals after treatment (usually every 2–3 months). Imaging sequences included T1-weighted precontrast and postcontrast (T1C+) and fluid-attenuated inversion recovery (FLAIR) MRI. The MRI data were exported to an iPlan workstation (Brainlab, Munich, Germany) from the electronic medical record. Using T1C+ MRI pre-LITT, immediately post-LITT, and at every follow-up, manual tumor segmentation was performed by a neuroradiologist to create a tridimensional volumetric measure. Similarly, manual segmentation of the edema was completed by a neuroradiologist, creating a tridimensional volumetric measure using FLAIR imaging data. On the preoperative scans, the tumor margin was the enhancing lesion. On the posttreatment scans, the margin was the ablation cavity. Single-volume measurements of each lesion and associated edema were calculated and verified by the senior author. The functional location of the lesions was classified per criteria described by Sawaya et al.[
Statistical analyses and data synthesis
Continuous variables were summarized with median and range; categorical variables, with frequency and percentage. Summary statistics were calculated for all variables. A Kaplan–Meier method was used to estimate overall survival (OS) and progression-free survival (PFS); survival curves were compared using a log-rank (Mantel-Cox) test. A Cox proportional hazards model with 95% confidence intervals was used to evaluate the difference in PFS between those patients who received post-LITT chemotherapy and those who did not. P < 0.05 was considered statistically significant for all analyses. Analyses were performed with SPSS 24 (IBM, Armonk, New York, USA). Graphs were constructed with the ggplot2 (
RESULTS
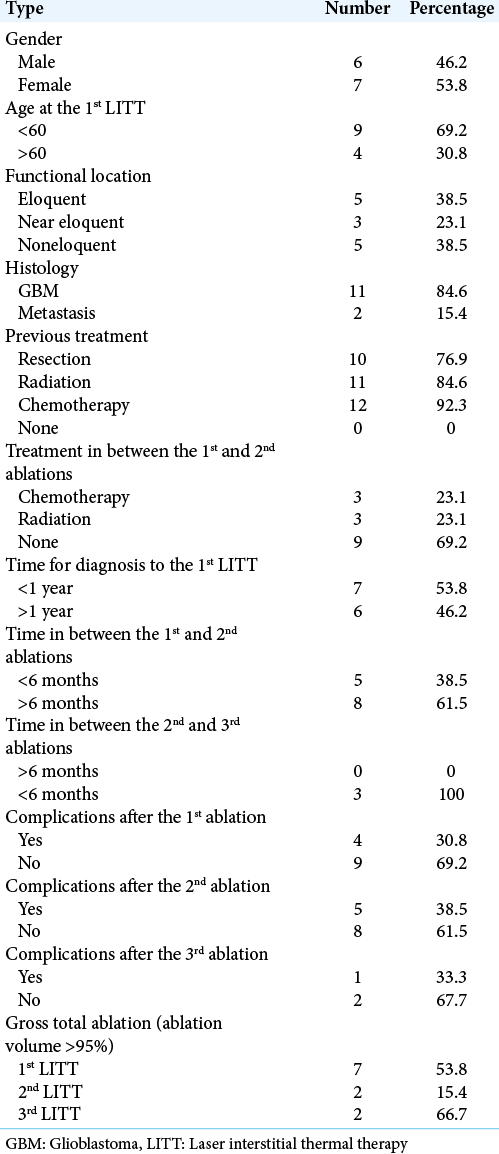
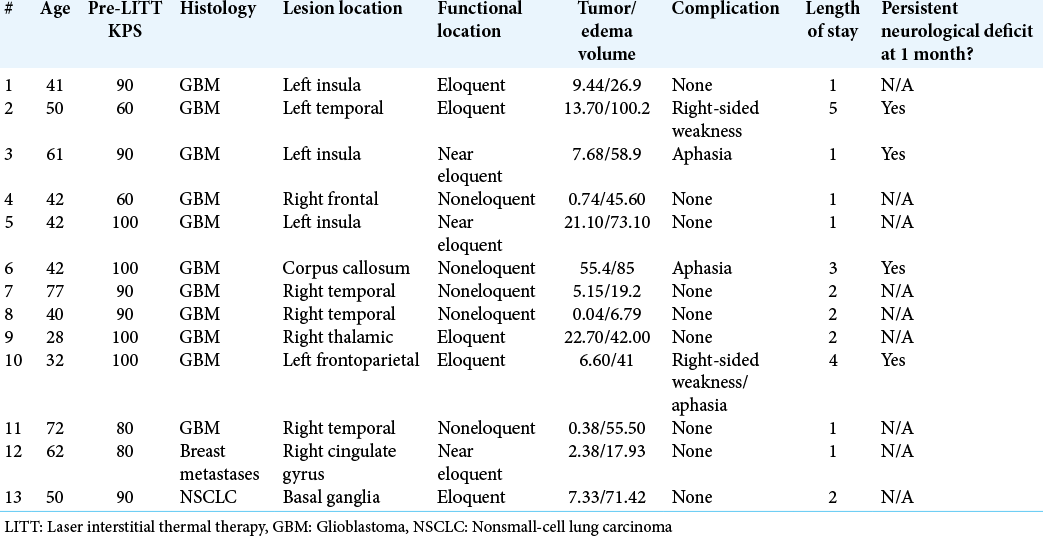
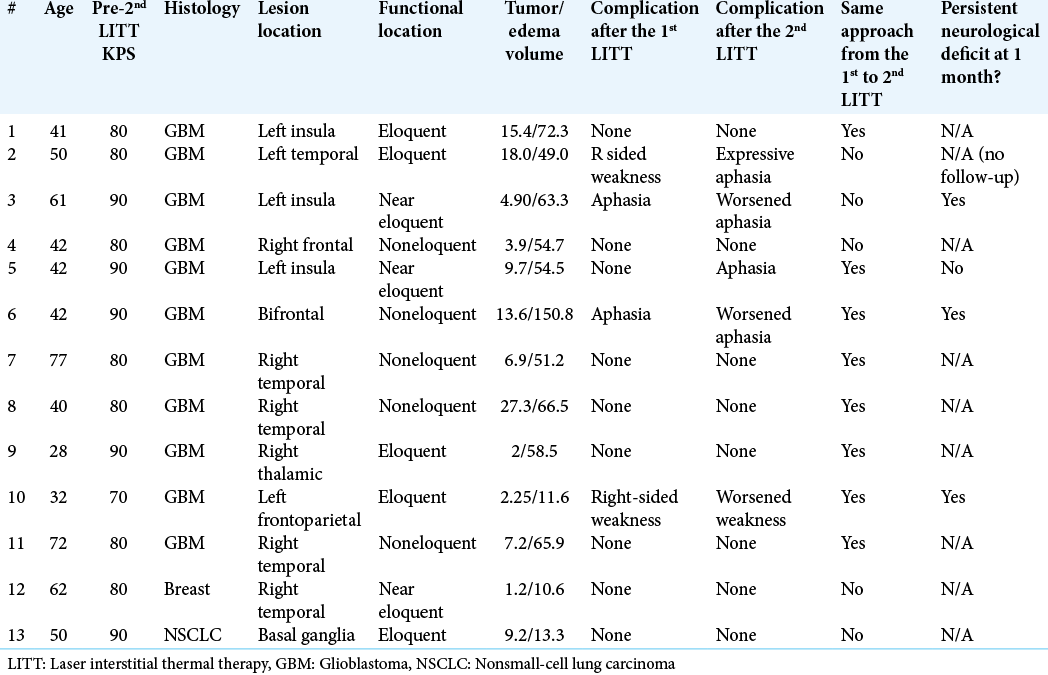
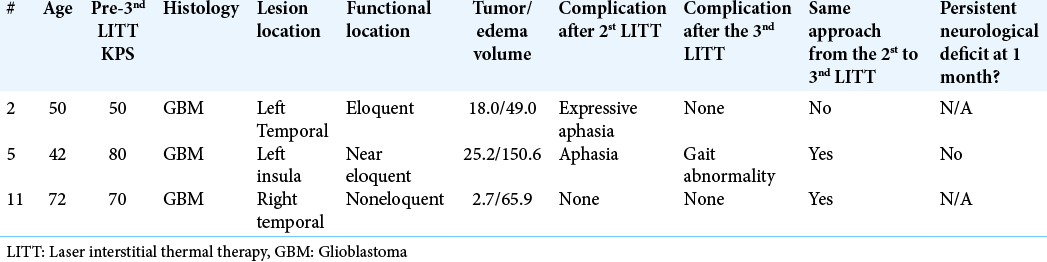
Patient and lesion characteristics are summarized in
GBM
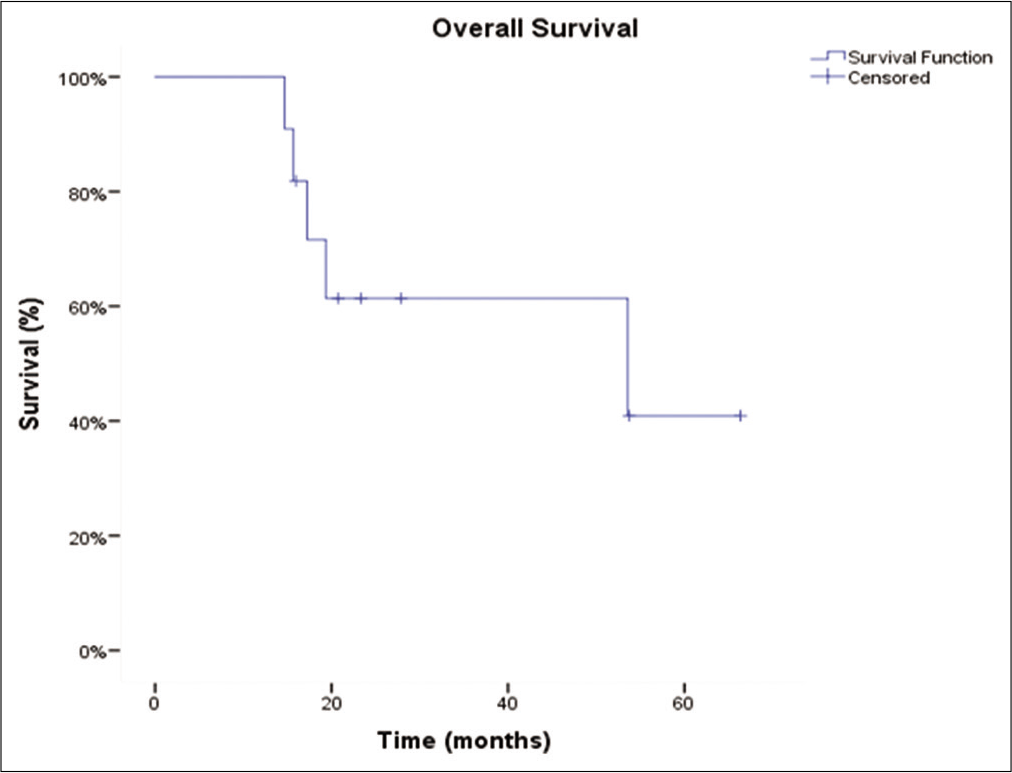
Of the 11 patients with GBMs that underwent repeated ablations for the tumors with local recurrence, eight had repeat laser ablations, whereas three underwent three ablations. The majority of GBM patients were IDH wild type (n = 8). Most tumors were located in either the temporal (n = 4) or frontal lobes (n = 3). Median time from diagnosis to the first LITT was 8 months, from the first LITT to second, 6.9 months, and 7.9 months from the second ablation to the third, respectively. Before the first LITT treatment, nine patients received radiation, eight received chemotherapy, and 10 underwent resection. Median OS from time of diagnosis was 66.7 months (confidence interval [CI] 95% 23.4–110.1). OS of the cohort is represented by a Kaplan–Meier curve in
Four patients (36%) experienced complications after the 1st LITT, 5 (45%) after the 2nd treatment, and 1 (33%) of the patients undergoing the 3rd LITT. The complications of all the patients after each ablation are summarized in
Metastases
Two patients underwent repeat ablations for brain metastases. One was a breast metastasis to the right temporal lobe and the other was a nonsmall-cell lung carcinoma (NSCLC) to the basal ganglia. The patient with the breast metastasis experienced no complications after either ablation and was alive with no recurrence at last follow-up, 5 years after the brain metastases diagnosis. The patient with the NSCLC metastasis also experienced no complications after either ablation and was alive with no recurrence at last follow-up, 6 years after diagnosis.
Complications
Four patients experienced complications after the first ablation, while five experienced complications after the second ablation. One of three patients experienced complications after the third ablation.
DISCUSSION
LITT is an emerging therapy for the treatment of intracranial malignancy, particularly for deep-seated and recurrent lesions. The precise, minimally invasive application of the probe with stereotactic guidance has been associated with reduced cost and length of hospital stay for the treatment of intracranial tumors.[
Despite these attributes, there is a paucity of data to support the use of repeated laser ablation for control of either primary or metastatic brain tumors. The earliest in the literature was a 2012 study by Jethwa et al.[
The median PFS for the GBM patients in our study after each ablation was 6.0, 3.2, and 2.1, respectively. However, these results are difficult to interpret given the heterogeneous nature of the cohort. A previous study examining repeat craniotomies for recurrent GBM patients found PFS of 7.8, 6.0, and 4.8, respectively.[
The complication rate for this cohort was slightly higher after the second LITT. Reports from the literature cite similar postoperative neurological morbidity after repeat resection.[
The results in this study should be interpreted in context of the salvage nature of the procedure. The patients selected for LITT in this study were not optimal surgical candidates. Across broad patient populations, LITT has not yet shown a significantly improved complication profile when compared to open craniotomy.[
Characteristics that excluded a craniotomy included medical comorbidities and depth and size of the tumor. For patients with medical comorbidities, LITT was chosen as a reasonable option to debulk the tumor while minimizing risk for medical complications associated with an open surgery and longer hospital stay. For patients with deep-seated tumors, LITT was chosen to preserve the access path tissue while still offering cytoreduction. For patients with small tumors, when craniotomy was determined not to justify the delay in adjuvant therapy, LITT was chosen to avoid delay starting chemotherapy. These are examples of the rationale used at our institution when selecting patients for LITT. Many patients exhibited a mixture of these characteristics.
The study is limited primarily by its small sample size and retrospective nature. Further, the variability in tumor histology and prior treatment obscures meaningful statistical analysis and conclusions. Future work should further stratify patient populations to study the efficacy and safety of LITT in specific patient subpopulations compared to open craniotomy. Despite these limitations, this study is the first of its kind to report on the safety of repeated LITT for GBM and brain metastases.
CONCLUSION
LITT is a burgeoning, minimally invasive approach for intracranial tumor ablation. The minimal morbidity associated with this technique allows it to be used safely for subsequent tumor recurrence and ablation, particularly in the context of GBM. Our study is the first to provide evidence for the safety profile of a dedicated cohort of patients with GBM and brain metastases receiving repeat LITT for local recurrence. Additional matched cohort studies are needed to confirm the efficacy of this treatment modality.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM. Laser ablation after stereotactic radiosurgery: A multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018. 130: 804-11
2. Barnett GH, Voigt JD, Alhuwalia MS. A systematic review and meta-analysis of studies examining the use of brain laser interstitial thermal therapy versus craniotomy for the treatment of high-grade tumors in or near areas of eloquence: An examination of the extent of resection and major complication rates associated with each type of surgery. Stereotact Funct Neurosurg. 2016. 94: 164-73
3. Beechar VB, Prabhu SS, Bastos D, Weinberg JS, Stafford RJ, Fuentes D. Volumetric response of progressing post-SRS lesions treated with laser interstitial thermal therapy. J Neurooncol. 2018. 137: 57-65
4. Biswas T, Okunieff P, Schell MC, Smudzin T, Pilcher WH, Bakos RS. Stereotactic radiosurgery for glioblastoma: Retrospective analysis. Radiat Oncol. 2009. 4: 11
5. Borghei-Razavi H, Koech H, Sharma M, Krivosheya D, Lee BS, Barnett GH. Laser interstitial thermal therapy for posterior fossa lesions: An initial experience. World Neurosurg. 2018. 117: e146-53
6. Chaunzwa TL, Deng D, Leuthardt EC, Tatter SB, Mohammadi AM, Barnett GH. Laser thermal ablation for metastases failing radiosurgery: A multicentered retrospective study. Neurosurgery. 2018. 82: 56-63
7. De Poorter J. Noninvasive MRI thermometry with the proton resonance frequency method: Study of susceptibility effects. Magn Reson Med. 1995. 34: 359-67
8. Eichberg DG, Menaker SA, Jermakowicz WJ. Multiple iterations of magnetic resonance-guided laser interstitial thermal ablation of brain metastases: Single surgeon’s experience and review of the literature. Oper Neurosurg (Hagerstown, Md.). 2019. 19: 204
9. Elliott RE, Parker EC, Rush SC, Kalhorn SP, Moshel YA, Narayana A. Efficacy of gamma knife radiosurgery for small-volume recurrent malignant gliomas after initial radical resection. World Neurosurg. 2011. 76: 128-40
10. Field KM, Simes J, Nowak AK, Cher L, Wheeler H, Hovey EJ. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol. 2015. 17: 1504-13
11. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009. 27: 4733-40
12. Hassaneen W, Levine NB, Suki D, Salaskar AL, de Moura Lima A, McCutcheon IE. Multiple craniotomies in the management of multifocal and multicentric glioblastoma. Clinical article. J Neurosurg. 2011. 114: 576-84
13. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: Single-institution series. Neurosurgery. 2013. 73: 1007-17
14. Hulsbergen AFC, Abunimer AM, Ida F, Kavouridis VK, Cho LD, Tewarie IA. Neurosurgical resection for locally recurrent brain metastasis. Neuro Oncol. 2021. 23: 2085-94
15. Hunn MK, Bauer E, Wood CE, Gasser O, Dzhelali M, Ancelet LR. Dendritic cell vaccination combined with temozolomide retreatment: Results of a phase I trial in patients with recurrent glioblastoma multiforme. J Neurooncol. 2015. 121: 319-29
16. Jethwa PR, Barrese JC, Gowda A, Shetty A, Danish SF. Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: Initial experience. Neurosurgery. 2012. 71: 133-44
17. Leuthardt EC, Voigt J, Kim AH, Sylvester P. A single-center cost analysis of treating primary and metastatic brain cancers with either brain laser interstitial thermal therapy (LITT) or craniotomy. Pharmacoecon Open. 2017. 1: 53-63
18. Mohammadi AM, Hawasli AH, Rodriguez A, Schroeder JL, Laxton AW, Elson P. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: A multicenter study. Cancer Med. 2014. 3: 971-9
19. Reardon DA, Schuster J, Tran DD, Fink KL, Nabors LB, Bota GL. ReACT: Overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. 2015. 33: 2009
20. Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998. 42: 1044-55
21. Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma an update. Crit Rev Oncol Hematol. 2016. 99: 389-408
22. Silva D, Sharma M, Barnett GH. Laser ablation vs open resection for deep-seated tumors: Evidence for laser ablation. Neurosurgery. 2016. 63: 15-26
23. Sughrue ME, Sheean T, Bonney PA, Maurer AJ, Teo C. Aggressive repeat surgery for focally recurrent primary glioblastoma: Outcomes and theoretical framework. Neurosurg Focus. 2015. 38: E11
24. Tang Y, Horikoshi M, Li WJ. Ggfortify: Unified interface to visualize statistical results of popular R packages. R J. 2016. 8: 474-89
25. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016. 41: E12
26. Traylor JI, Patel R, Habib A, Muir M, de Almeida Bastos DC, Rao G. Laser interstitial thermal therapy to the posterior fossa: Challenges and nuances. World Neurosurg. 2019. 132: e124-32
27. Wickham H.editors. Ggplot2: Elegant Graphics for Data Analysis. Berlin: Springer; 2016. p.