- Department of Neurosurgery, Faculty of Medicine, Tanta University, Tanta, Egypt
- Department of Neurology, Faculty of Medicine, Cairo University, Cairo, Egypt
- Department of Radiology, Ministry of Health, Cairo, Egypt
- Department of Clinical Neurophysiology, Faculty of Medicine, Cairo University, Cairo, Egypt
- Department of Anesthesia, Surgical Intensive Care and Pain Management, Zagazig, Egypt.
- Department of Neurosurgery, Faculty of Medicine, Zagazig University, Zagazig, Egypt.
Correspondence Address:
Hussein Hamdi, Department of Neurosurgery, Faculty of Medicine, Tanta University, Tanta, Egypt.
DOI:10.25259/SNI_1081_2022
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Hussein Hamdi1, Nirmeen Kishk2, Reham Shamloul2, Mona K. Moawad2, Micheal Baghdadi3, Mina Rizkallah3, Amani Nawito4, Mohammad Edrees Mohammad2, Hatem Nazmi5, Yasser Mohamed Nasr5, Salwa Hassan Waly5, Mo’men Elshahat6, Rehab Magdy2, Alshimaa S. Othman2, Hesham Nafea4, Amro M Fouad2, Ismail Elantably6, Haytham Rizk2, Enas Elsayyad2, Ahmed A. Morsy6. Resective epilepsy surgery in a limited-resource settings: A cohort from a multi-disciplinary epilepsy team in a developing country. 14-Jul-2023;14:240
How to cite this URL: Hussein Hamdi1, Nirmeen Kishk2, Reham Shamloul2, Mona K. Moawad2, Micheal Baghdadi3, Mina Rizkallah3, Amani Nawito4, Mohammad Edrees Mohammad2, Hatem Nazmi5, Yasser Mohamed Nasr5, Salwa Hassan Waly5, Mo’men Elshahat6, Rehab Magdy2, Alshimaa S. Othman2, Hesham Nafea4, Amro M Fouad2, Ismail Elantably6, Haytham Rizk2, Enas Elsayyad2, Ahmed A. Morsy6. Resective epilepsy surgery in a limited-resource settings: A cohort from a multi-disciplinary epilepsy team in a developing country. 14-Jul-2023;14:240. Available from: https://surgicalneurologyint.com/surgicalint-articles/12433/
Abstract
Background: Multidisciplinary pre-surgical evaluation is vital for epilepsy surgery decision and outcomes. Resective epilepsy surgery with assisted monitoring is currently a standard treatment for focal drug resistant epilepsy (DRE). In resource-limited countries, lack of epilepsy surgery center is a huge challenge. We presented and illustrated how to create a multidisciplinary protocol with resource-limited settings in a developing country and epilepsy surgery outcome using brain mapping and monitoring techniques for ensuring satisfactory resection.
Methods: We created multicentric incomplete but complementary units covering all epilepsy-related sub-specialties and covering a wide geographical area in our country. Then, we conducted a prospective and multicentric study with low resource settings on patients with focal DRE, who underwent resective epilepsy surgery and were followed up for at least 12 months and were evaluated for postoperative seizure outcome and complications if present. Preoperative comprehensive clinical, neurophysiological, neuropsychological, and radiological evaluations were performed by multidisciplinary epilepsy team. Intraoperative brain mapping including awake craniotomy and direct stimulation techniques, neurophysiological monitoring, and electrocorticography was carried out during surgical resection.
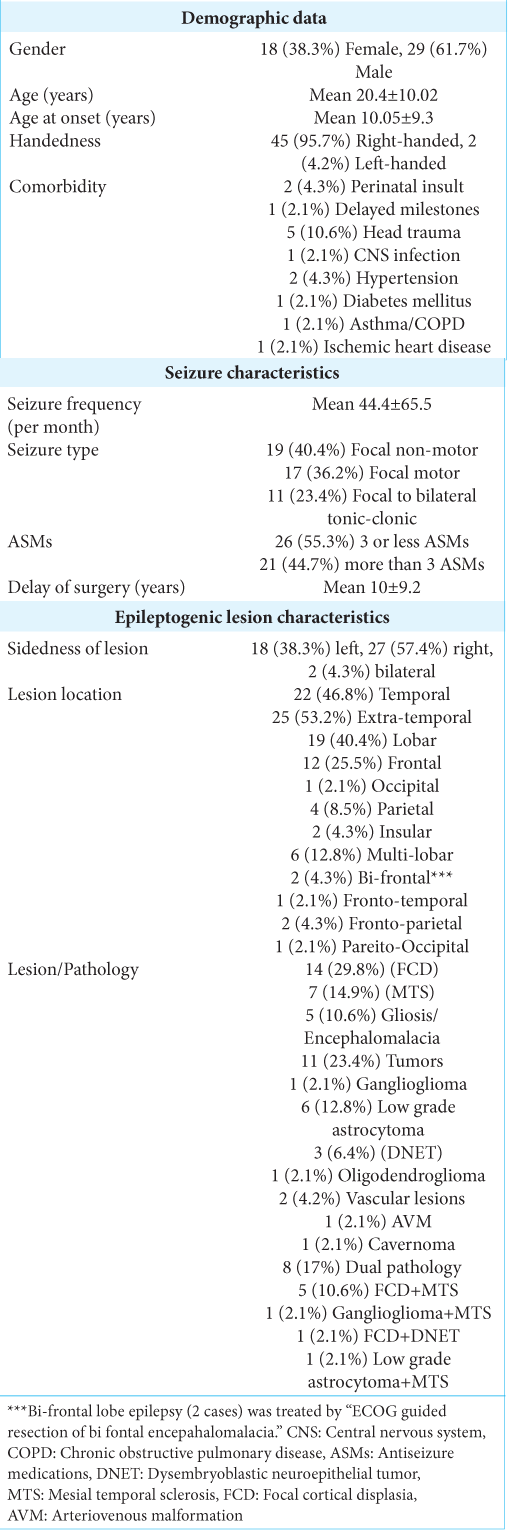
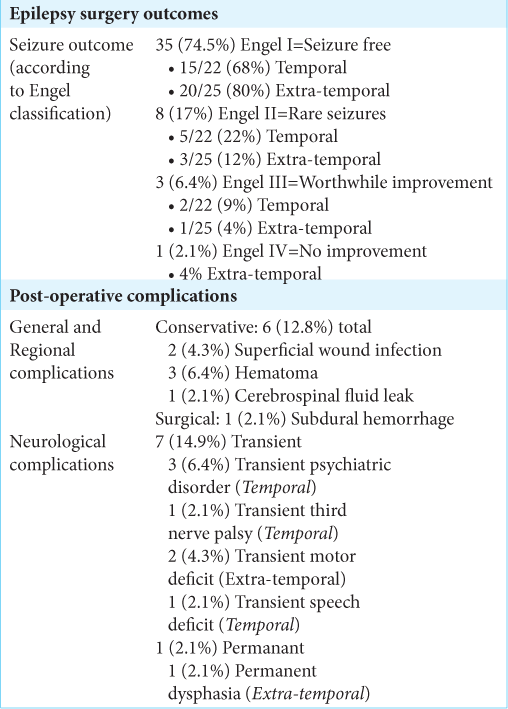
Results: The study included 47 patients (18 females and 29 males) with mean age 20.4 ± 10.02 years. Twenty-two (46.8%) patients were temporal epilepsy while 25 (53.2%) were extra-temporal epilepsy. The epilepsy surgery outcome at the last follow up was Engel Class I (seizure free) in 35 (74.5%), Class II (almost seizure free) in 8 (17%), Class III (worthwhile improvement) in 3 (6.4%), and Class IV (no worthwhile improvement) in 1 patient (2.1%).
Conclusion: With low resource settings and lack of single fully equipped epilepsy center, favorable outcomes after resective surgery in patients with focal DRE could be achieved using careful presurgical multidisciplinary selection, especially with using intraoperative brain mapping and electrocorticography techniques.
Keywords: Developing country, ECoG, Epilepsy, Resective, Surgery
INTRODUCTION
Seizure freedom is the ultimate final goal of any management strategy for epilepsy patients. Delayed proper treatment or failure of seizure control will be associated with progressive decline of several cognitive and psychiatric aspects with critical impact on the quality of life.[
Recently, several surgical techniques and approaches have been proposed for this group of patients suffering intractable epilepsy.[
On the other hand, the usual cost of such complicated techniques could obstacle these procedures in the developing countries. Adaptation and selection among such expensive surgical techniques should be carefully discussed and applied with special attention to efficacy and available resources at the same time in a wide geographical area.
We present our multicentric prospective cohort in a developing country with special focus on surgical and resource-limited techniques utilized to optimize epilepsy surgery outcome in intractable focal epilepsy.
MATERIALS AND METHODS
Study design
We conducted a prospective study analysis in three tertiary hospitals and referral centers in our country to cover a wider geographical area between January 2018 and May 2021 [
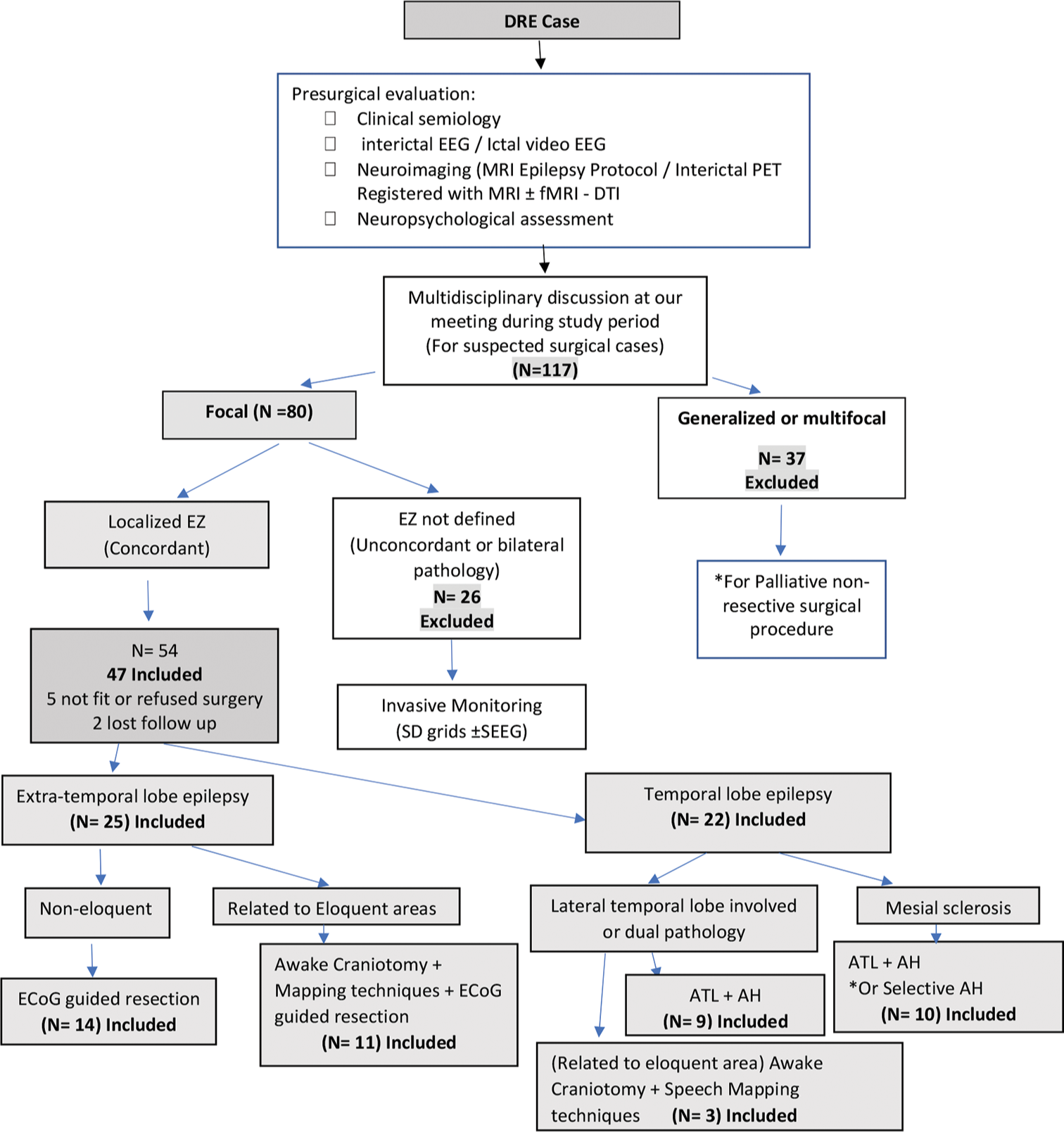
Figure 1:
Flowchart shows patients inclusion for resective epilepsy surgery. DRE: Drug-resistent epilepsy, EEG: Electro-encephalography, PET: Positive emission tomogrpahy, MRI: Magnetic resonance image, DTI: Diffusion tensor imaging, N: Number of patients, SD: Subdural, SEEG: Stereotactic encephalography, ATL: Anterior temporal lobectomy, AH: Amygdalohippocampectomy, ECoG: Electro-enephalography, EZ: Epilepstic zone. *According to each case.
Different variables were analyzed to be correlated with the clinical Engel class outcome such as gender, age of onset of the disease, duration, age at surgery, handedness, ASMs load, and seizure frequency per month, location in the hemisphere, dominant hemisphere, and pathology of the lesion.
Epilepsy center and multidisciplinary team
Lack of resources and trained staff in a single center was the main obstacle toward starting a specialized epilepsy surgery program. It was also extremely difficult to establish all resources required in a single center. A multidisciplinary board from different universities was formed after cooperation between several Neurology and Neurosurgery departments, and Specialized Neuroradiology team. The team included trained highly specialized epileptologists, neurophysiology experts, epilepsy neuroradiologists, nuclear medicine specialists, neuropsychologists, epilepsy neurosurgeons with anesthesiologist, and neurophysiology specialist who is expert in intraoperative brain mapping techniques and electrocorticography (ECOG) recordings. The team members joined a weekly real or on-line video conference meeting to discuss all candidate cases with focal DRE regarding the clinical, imaging, and electrophysiological data. The team was in contact with three international leading experts in epilepsy. Surgical decision was always discussed through the multidisciplinary board considering all benefits and risks to be explained to the patient before surgery and during the informed consent.
The surgical theaters in all different hospitals were equipped with all standard surgical instruments and devices required. Portable machines such as intraoperative navigation machine and neurophysiological monitoring device for mapping and ECoG recording were always carried and used in our different centers.
Preoperative workup
All patients were subjected to the following
Detailed history
Demographics and clinical data were documented that included age, gender, age of onset, duration of epilepsy, and ASMs regimen. Seizure semiology was classified according to the new ILAE classification.[
Video EEG
All recruited patients were scheduled for ictal video EEG recording with the help of a well-trained EEG technician. Arrangement ASMs withdrawal before ictal recording was done based on seizure frequency, type, and dose of ASMs. Rapid ASMs withdrawal up to 30% of daily dose with sleep deprivation before EEG recording to shorten the duration to first seizure.[
Neuropsychological assessment[ 1 ]
Patients were screened for depression and anxiety by Beck depression inventory questionnaire – Arabic version[
Figure 3:
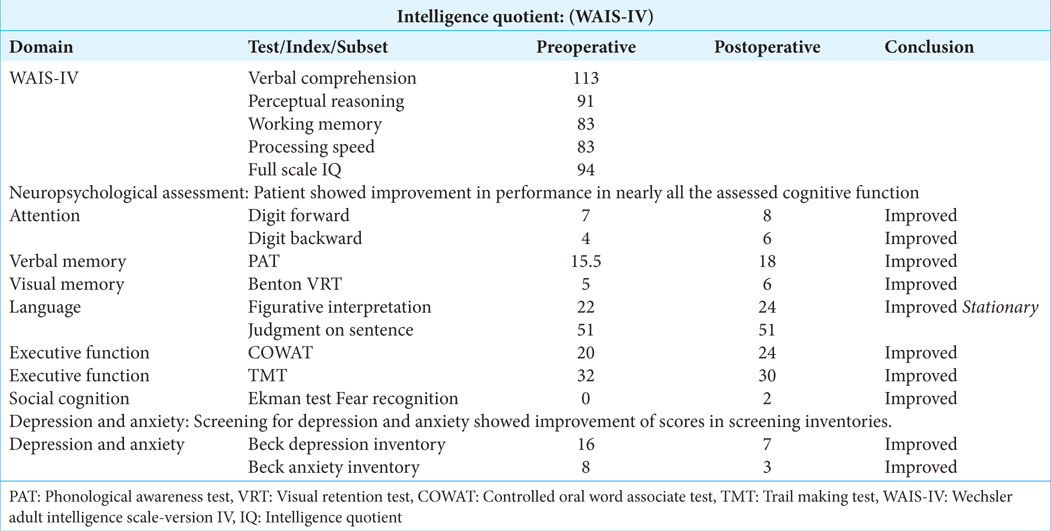
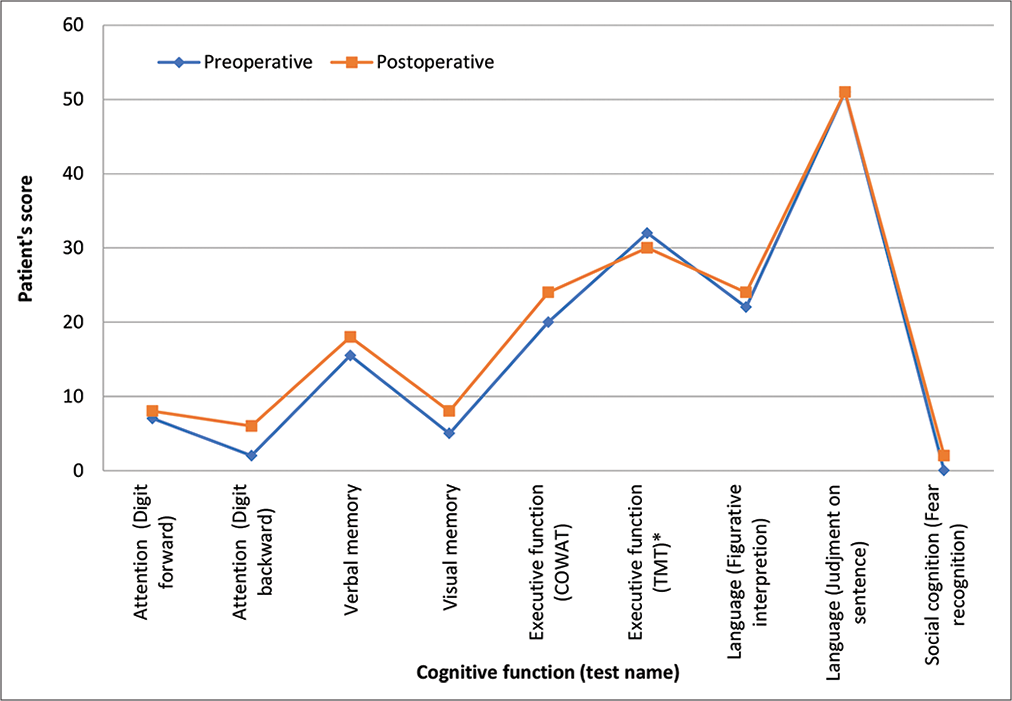
Comparison of Patient’s performance (showed in Figure 4) in preoperative and postoperative neuropsychological assessment that showed improvement of the scores in postoperative performance. Unlike the rest of tests, the lower the score is better; however, preoperative and postoperative score was within normal range. COWAT: The Controlled Oral Word Association Test. TMT: The Trail Making Test. * TMT-A uses all numbers, TMT-B alternates numbers and letters, in consecutive order.
Neuroimaging techniques
We installed a neuroimaging protocol for epilepsy patients with acquisition specifications to catch various epileptic lesions and relevant pathology. Within four referral radiology centers, we launched the imaging protocol to cover wider geographical areas in our country. These centers were in several major cities. Epileptologists in different hospitals were announced about this protocol to follow it before referring patients to our team.
Magnetic resonance imaging (MRI) brain (epilepsy protocol)
All subjects were scanned on a 1.5 Tesla GESIGNA closed-configuration whole body scanner using a standard quadrature head coil. We acquired isotropic Sagittal 3D T1-weighted spoiled gradient, sagittal Cube T2 FLAIR, 2D Axial T2, Coronal T2 perpendicular to the long axis of the hippocampus, diffusion-weighted imaging, and susceptibility-weighted scan. In addition, we added complementary T1 MRI with gadolinium and/or thin cuts coronal T2 (2 mm) covering the temporal pole and sphenoid bones if needed.
Imaging postprocessing
FreeSurfer software package version 6.0 (https://surfer.nmr. mgh.harvard.edu/) was used for the volumetric analysis. Every patient was provided with an individualized report that detailed the volume and percentile of each segmented region compared to age-matched normative database. 3D slicer version 4.11 was used to generate brain surface 3D models to guide the surgeon intraoperatively according to brain surface anatomical curvatures [
Figure 4:
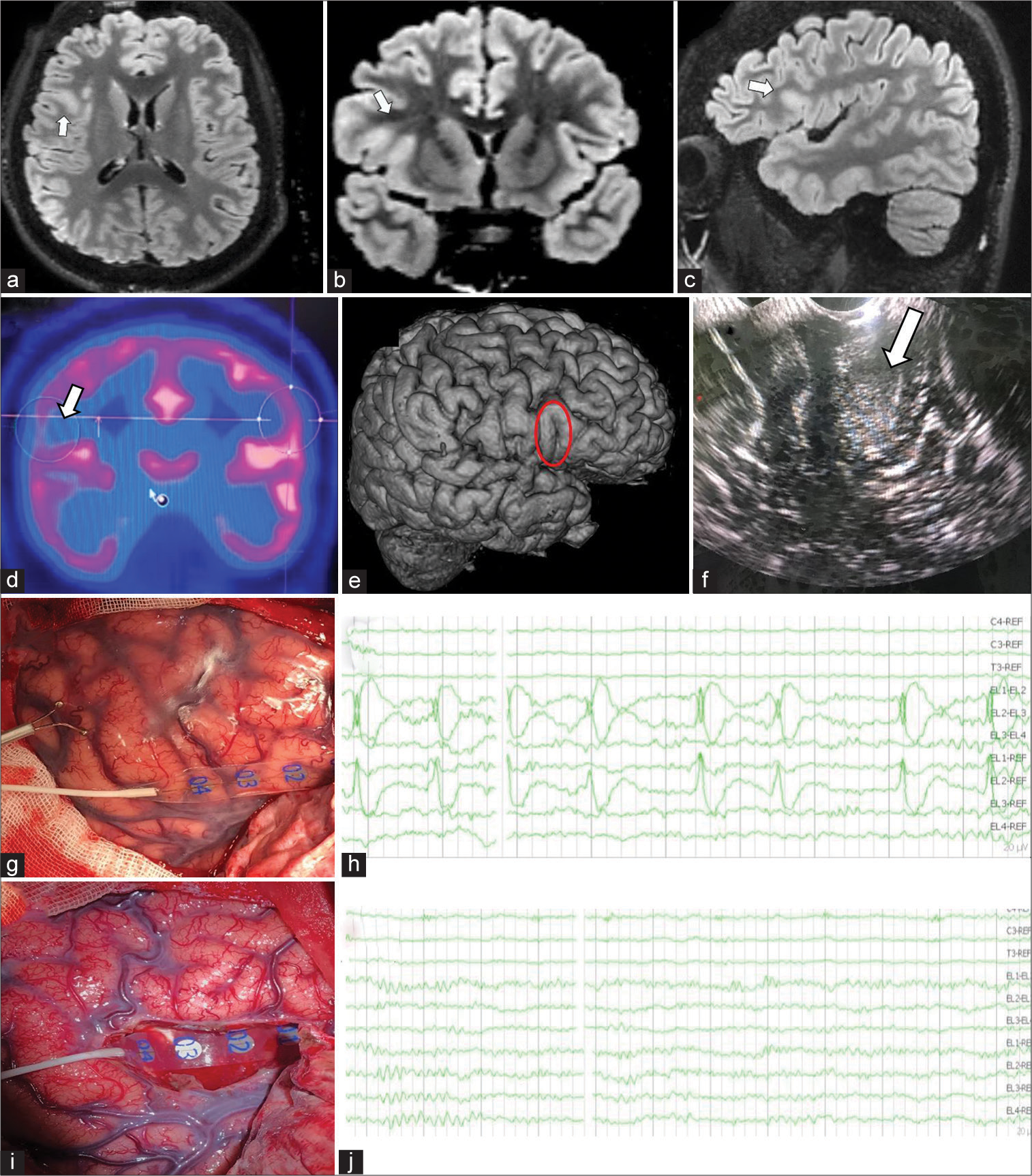
A 20-years-old male patient with focal drug resistant epilepsy. Patient was enrolled to our proposed presurgical evaluation protocol. Seizure Semiology was focal aware motor seizures in the form of palpitation, sense of fear followed by left-sided tonic posture with vocalization rarely with or Focal to bilateral tonic clonic seizure, with an ictal EEG onset in right frontal derivation. This was concordant with neuroimaging which revealed right inferior frontal focal cortical dysplasia. Patient underwent resective surgery using intraoperative mapping techniques and electrocorticography (ECoG), with Engel Class I outcome. Comparison of Patient’s performance in preoperative and postoperative neuropsychological assessment showed improvement of the scores in postoperative performance.(Supplementary Data) (a,-c): Axial, coronal, and sagittal magnetic resonance (MR) neuroimaging shows right inferior frontal focal cortical dysplasia in the form of cortical thickening and blurring of grey-white matter junction with transmantle sign (highlighted by arrow). (d) Positron emission tomography image shows the interictal hypometabolism hypo-metabolism area (arrow) corresponding to the location of the focal cortical dysplasia. (e) Preoperative 3-dimentional illustration planning planning model shows the lesion (circle). (f) Intraoperative ultrasonography image shows thickened and hyper-echoic dysplasia (highlighted by arrow). (g and h) Intraoperative photograph shows preresection ECoG strip over the surgical target, and recording screenshot shows corresponding rhythmic sharp waves. (i and j) Intraoperative photograph shows postresection image with ECoG strip over the insula in the depth of resection cavity, and recording screenshot shows postresection ECoG recording shows normal electroencephalography findings.
Positron emission tomography (PET)/computed tomography (CT)
Fluorodeoxyglucose (FDG) brain PET/CT imaging protocol is well described in Society of Nuclear Medicine and European Association of Nuclear Medicine guidelines.[
PET/MRI co-registration
PET images were registered to the magnetic resonance (MR) images using FSL version 5.0.11 (using FLIRT toolbox).
Anesthesia and surgical techniques
Type of anesthesia, either awake or general anesthesia (GA), was depending on proximity of EZ to an eloquent area according to our protocol [
Intraoperative ECoG was performed in all our patients using portable 64-channel intraoperative neurophysiological monitoring device, either (ISIS, IOM system, INOMED, Inc.) or (NIM Eclipse, Medtronic, Minneapolis, Minnesota). Different strip and grid electrodes were used to cover suspected epileptogenic area. Pre and post resection ECOG recordings were done by single experienced neurophysiologist and blinded from the location of electrode during surgery to properly define EZ and confirm satisfactory resection [
Standard microsurgical resection techniques were performed either for awake or GA cases. Intraoperative ultrasonography (EUB-405 plus ultrasound scanner, HITACHI) and intraoperative navigation technique (Brainlab, Germany) were used in all cases for locating lesions and previously defined EZ with evaluating the extent of resection.
Follow-up
All patients were planned to be assessed immediately postoperatively with clinical and neurological evaluation with CT/MR follow up to role out any immediate surgical complications. Discharge from hospital was usually in the 3rd postoperative day. The follow-up was scheduled to be within 2 weeks in the neurosurgical clinic, 1 month with the multidisciplinary clinic, then with the epileptologist at the 3rd month to assess the outcome and ASMs modifications and withdrawal, then 6 months and 1 year after surgery then yearly. Engel classification for epilepsy surgery outcome was the standard method to evaluate our patients in the last clinical visit with at least 1-year follow-up.
Statistical analysis
All the statistical and descriptive analysis were performed by SPSS version 19 (IBM, USA). The normality distribution was tested by Kolmogorov–Smirnov test of normality. Because of the non-normality distribution of some data, we used Kendall’s tau_b correlation one-sided test. Categorical variables were tested using Chi-square test. Benferroni correction was performed to limit the Type II error during multiple hypothesis testing.
RESULTS
Patients’ characteristics
We included 54 patients with focal lesioned epilepsy, five refused surgical management, and we lost two cases for postsurgical follow-up then finally the study included 47 (18 females and 29 males) while seven patients were either refusing surgery, not fit for surgery, or lost during the follow up [
EZ
According to the laterality of the epileptic lesion, there were 18 (38.3%) on the left side, 27 (57.4%) on the right side, and bilateral in two lesions (4.3%). The location of the lesion was temporal in 22 (46.8%), extra-temporal in 25 (53.2%); 19 (40.4%) lobar, and multilobar in 6 (12.8%). The nature of the epileptic tissue was highly variable in our cohort as mentioned in
Surgical outcome
The epilepsy surgery outcome at the last follow-up (12– 51 months) was Engel Class I (seizure free) in 35 (74.5%), Class II (almost seizure free) in 8 (17%), Class III (worthwhile improvement) in 3 (6.4%), and Class IV (no worthwhile improvement) in 1 patient (2.1%). Surgical outcome and complications are mentioned in
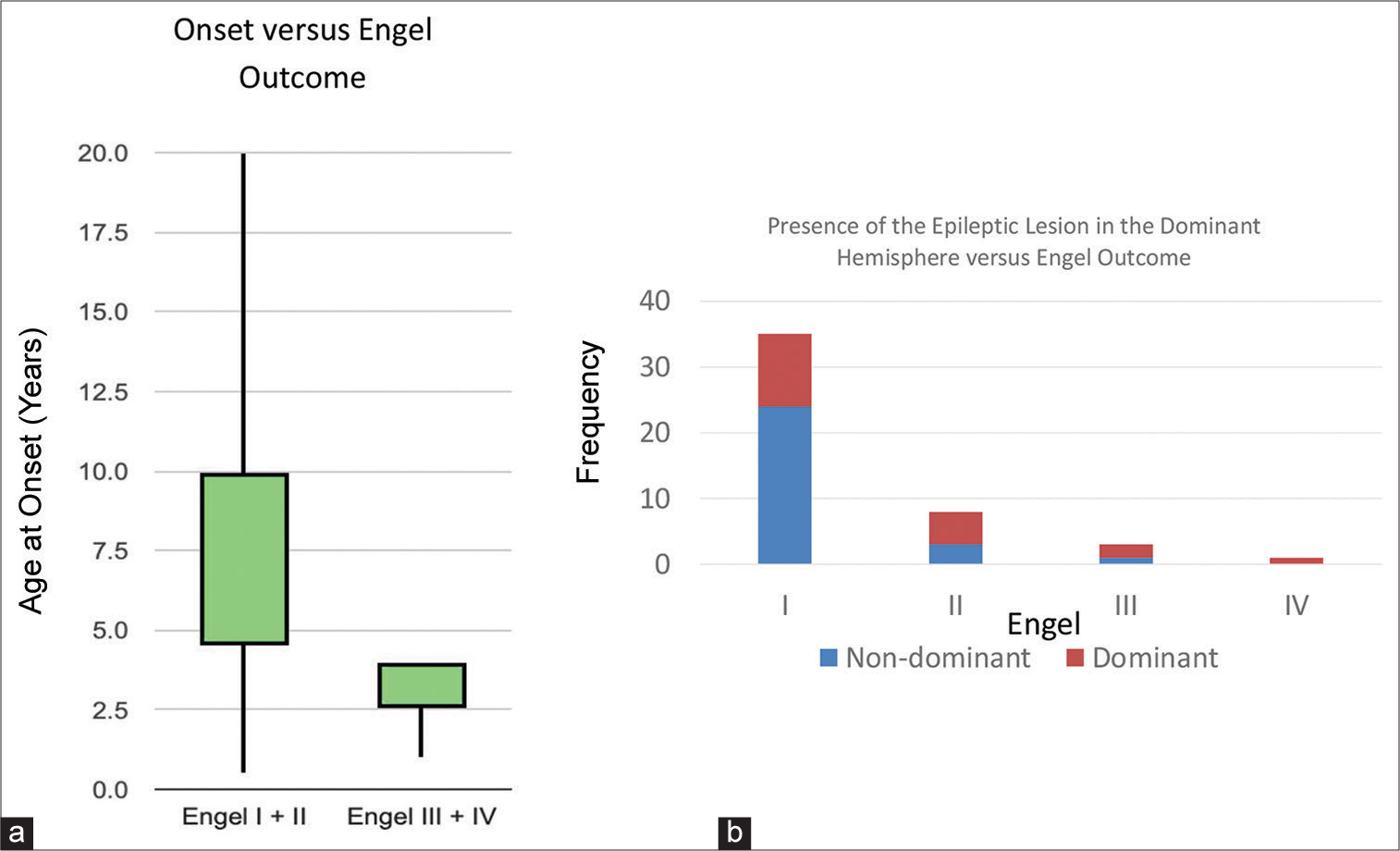
Different variables were analyzed to be correlated with the clinical Engel class outcome. The presence of the epileptic lesion in the non-dominant hemisphere and late-onset disease was the main factors giving us better Engel outcome in our study [
DISCUSSION
Approximately one-third of epileptic patients have seizures that are refractory to ASM. Left untreated, patients are at risk of developing several comorbidities, and potentially death.[
Non-invasive video-EEG monitor and cranial MRI are highly conclusive regarding resective surgery throughout a larger proportion of patients (~ 60%), invasive exploration plays a pivotal role for the remainder [
The technique of ECoG, the intraoperative recording of cortical potentials was pioneered by Penfield and Jasper in the early 1950s to map focal interictal spiking and to determine the extent of the resection.[
The advantages of the ECoG are the flexible placement of electrodes, performed before and after each stage of resection, and direct electrical stimulation of the regions involved in functions to be spared by the resection can be delineated with a high degree of confidence. The limitations are the limited sampling time, spontaneous epileptiform activity consists exclusively of interictal spikes and sharp waves, and seizures are rarely recorded, it is impossible to distinguish primary epileptiform discharges from secondarily propagated discharges arising at a distant epileptogenic site, both the background activity and epileptiform discharges may be altered by the anesthetics and by the surgery itself.[
Furthermore, our statistical model succeeded to find some preoperative factors associated with favorable outcome. In current study, late age of onset of epilepsy was a favorable factor to achieve better Engel outcome class which was clearly showed in some other epilepsy studies.[
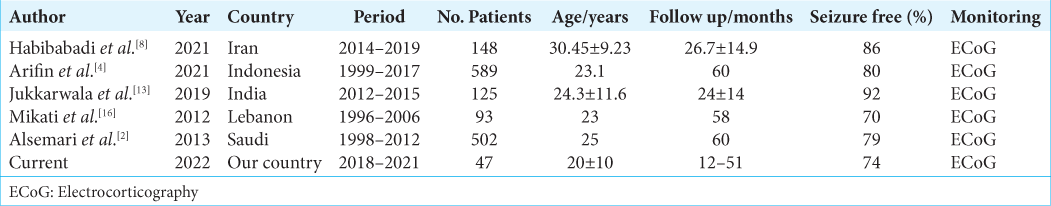
We reviewed the publications[
CONCLUSION
Resective surgery and satisfactory outcome in focal epilepsy could be achieved using presurgical multidisciplinary selection, especially with intraoperative neurophysiological and electrocorticography techniques. It is a major treatment option, even with low resources settings and should be encouraged in developing countries.
Ethical consideration
The authors have nothing to declare regarding any kind of conflicts of interest or financial issue.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgment
The authors would like to present the gratitude and to be grateful for the great help and the ultimate support in decision-making process given to the study from the three international leading epilepsy experts; Professor Bertil Rydenhag from Gothenburg University, Sweden Professor Mohamad Koubeissi from George Washington University, USA and Professor Ahmed Abdelmoity from University of Missouri-Kansas City, School of Medicine USA.
References
1. Alarcón G, Valentín A, Watt C, Selway RP, Lacruz ME, Elwes RDC. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging?. J Neurol Neurosurg Psychiatry. 2006. 77: 474-80
2. Alsemari A, Al-Otaibi F, Baz S, Althubaiti I, Aldhalaan H, Macdonald D. Epilepsy surgery series: A study of 502 consecutive patients from a developing country. Epilepsy Res Treat. 2014. 2014: 286801
3. Al-Shatti TS. Psychometric properties of the arabic version of the beck anxiety inventory in the State of Kuwait. J Educ Psychol Sci. 2015. 16: 431-63
4. Arifin MT, Hanaya R, Bakhtiar Y, Bintoro AC, Iida K, Kurisu K. Initiating an epilepsy surgery program with limited resources in Indonesia. Sci Rep. 2021. 11: 5066
5. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988. 56: 893-7
6. Engel J. Approaches to refractory epilepsy. Ann Indian Acad Neurol. 2014. 17: S12-7
7. Fawzi MH, Fawzi MM, Abu-Hindi W. Arabic version of the Major Depression Inventory as a diagnostic tool: Reliability and concurrent and discriminant validity. East Mediterr Health J. 2012. 18: 304-10
8. Habibabadi JM, Moein H, Jourahmad Z, Ahmadian M, Basiratnia R, Zare M. Outcome of epilepsy surgery in lesional epilepsy: Experiences from a developing country. Epilepsy Behav. 2021. 122: 108221
9. Hamdi H, Albader F, Spatola G, Laguitton V, Trebuchon A, Bartolomei F. Long-term cognitive outcome after radiosurgery in epileptic hypothalamic hamartomas and review of the literature. Epilepsia. 2021. 62: 1369-81
10. Harroud A, Bouthillier A, Weil AG, Nguyen DK. Temporal lobe epilepsy surgery failures: A review. Epilepsy Res Treat. 2012. 2012: 201651
11. Holdnack JA, Zhou X, Larrabee GJ, Millis SR, Salthouse TA. Confirmatory factor analysis of the WAIS-IV/WMS-IV. Assessment. 2011. 18: 178-91
12. Hou Z, Duan QT, Ke YY, An N, Yang H, Liu SY. Predictors of seizure freedom in patients undergoing surgery for central nervous system infection-related epilepsy: A systematic review and meta-analysis. Front Neurol. 2021. 12: 668439
13. Jukkarwala A, Baheti NN, Dhakoji A, Salgotra B, Menon G, Gupta A. Establishment of low cost epilepsy surgery centers in resource poor setting. Seizure. 2019. 69: 245-50
14. Kirby J, Leach VM, Brockington A, Patsalos P, Reuber M, Leach JP. Drug withdrawal in the epilepsy monitoring unit-The patsalos table. Seizure. 2020. 75: 75-81
15. Kuruvilla A, Flink R. Intraoperative electrocorticography in epilepsy surgery: Useful or not?. Seizure. 2003. 12: 577-84
16. Mikati MA, Ataya N, El-Ferezli J, Shamseddine A, Rahi A, Herlopian A. Epilepsy surgery in a developing country (Lebanon): Ten years experience and predictors of outcome. Epileptic Disord. 2012. 14: 267-74
17. Mullin JP, Shriver M, Alomar S, Najm I, Bulacio J, Chauvel P. Is SEEG safe? A systematic review and meta-analysis of stereo-electroencephalography-related complications. Epilepsia. 2016. 57: 386-401
18. Nasr YM, Waly SH, Morsy AA. Scalp block for awake craniotomy: Lidocaine-bupivacaine versus lidocainebupivacaine with adjuvants. Egypt J Anaesth. 2020. 36: 7-15
19. Rössler K, Sommer B, Grummich P, Hamer HM, Pauli E, Coras R. Risk reduction in dominant temporal lobe epilepsy surgery combining fMRI/DTI maps, neuronavigation and intraoperative 1.5-Tesla MRI. Stereotact Funct Neurosurg. 2015. 93: 168-77
20. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017. 58: 512-21
21. Tsuchida TN, Acharya JN, Halford JJ, Kuratani JD, Sinha SR, Stecker MM. American clinical neurophysiology society: EEG guidelines introduction. J Clin Neurophysiol. 2016. 33: 301-2
22. Vakharia VN, Duncan JS, Witt JA, Elger CE, Staba R, Engel J. Getting the best outcomes from epilepsy surgery. Ann Neurol. 2018. 83: 676-90
23. Van Rijckevorsel K. Cognitive problems related to epilepsy syndromes, especially malignant epilepsies. Seizure. 2006. 15: 227-34
24. Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009. 36: 2103-10
25. Wilson SJ, Baxendale S, Barr W, Hamed S, Langfitt J, Samson S. Indications and expectations for neuropsychological assessment in routine epilepsy care: Report of the ILAE Neuropsychology Task Force, Diagnostic Methods Commission, 2013-2017. Epilepsia. 2015. 56: 674-81