- Department of Neurosurgery, University of Toyama, Toyama, Japan.
Correspondence Address:
Satoshi Kuroda, Department of Neurosurgery, University of Toyama, Toyama, Japan.
DOI:10.25259/SNI_173_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yuichiro Koga, Shusuke Yamamoto, Satoshi Kuroda. Resolution of white matter hyperintensity after surgical revascularization in moyamoya disease – A report of three cases. 12-Apr-2024;15:131
How to cite this URL: Yuichiro Koga, Shusuke Yamamoto, Satoshi Kuroda. Resolution of white matter hyperintensity after surgical revascularization in moyamoya disease – A report of three cases. 12-Apr-2024;15:131. Available from: https://surgicalneurologyint.com/surgicalint-articles/12851/
Abstract
Background: Moyamoya disease often presents white matter hyperintensity (WMH) lesions on fluid-attenuated inversion recovery (FLAIR) images, which is generally accepted as irreversible. We, herein, describe three cases of moyamoya disease with WMH lesions that regressed or disappeared after surgical revascularization.
Case Description: This report included two pediatric and one young adult case that developed transient ischemic attacks or ischemic stroke due to bilateral Moyamoya disease. Before surgery, five of their six hemispheres had WMH lesions in the subcortical and/or periventricular white matter on FLAIR images. The lesions included morphologically two different patterns: “Striated” and “patchy” morphology. In all of them, combined bypass surgery was successfully performed on both sides, and no cerebrovascular events occurred during follow-up periods. On follow-up magnetic resonance examinations, the “striated” WMH lesions completely disappeared within six months, while the “patchy” WMH lesions slowly regressed over 12 months.
Conclusion: Based on radiological findings and the postoperative course of the WMH lesions, the “striated” WMH lesions may represent the inflammation or edema along the neuronal axons due to cerebral ischemia, while the “patchy” WMH lesions may represent vasogenic edema in the white matter through the blood-brain barrier breakdown. Earlier surgical revascularization may resolve these WMH lesions in Moyamoya disease.
Keywords: Cerebral ischemia, Moyamoya disease, Revascularization, White matter hyperintensity
INTRODUCTION
Moyamoya disease is characterized by chronic, progressive stenosis of the terminal portion of internal carotid arteries (ICAs) and its main branches, and the anterior and middle cerebral arteries, respectively.[
CASE PRESENTATION
Case 1
A 13-year-old girl presented with transient weakness of the right extremities. Neurological examinations on admission revealed no neurological deficits. Cerebral angiography showed severe stenosis of the terminal portion of the ICA on both sides. The perforating arteries were markedly dilated [
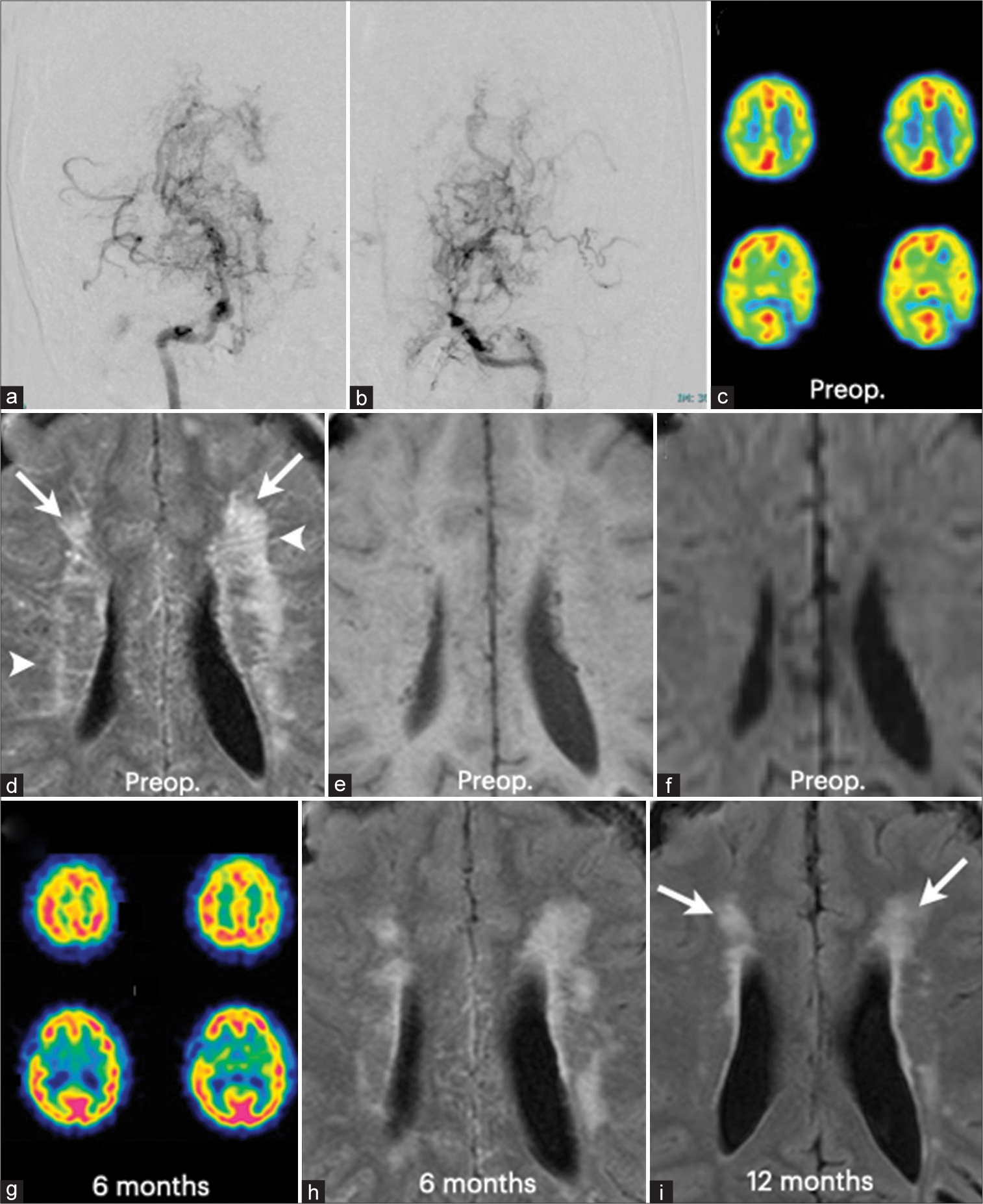
Figure 1:
Radiological findings of a 13-year-old girl with moyamoya disease (Case 1). (a) Right internal carotid angiography demonstrated typical findings of moyamoya disease, including severe stenosis of the supraclinoid portion of the internal carotid artery and the horizontal portions of the anterior and middle cerebral arteries. (b) Left internal carotid angiography also showed very similar findings. Note the markedly dilated perforating arteries on both sides. (c) Single photon emission computed tomography (SPECT) demonstrated cerebral blood flow reduction in the internal carotid artery territories on both sides. (d) Fluid-attenuated inversion recovery (FLAIR) image showed the “patchy” hyperintensity lesions (arrows) in the periventricular white matter and also the “striated” hyperintensity lesions (arrowheads) in the periventricular white matter. The “striated” lesions appear to radiate from the lateral ventricles. (e) These hyperintensity lesions on FLAIR image cannot be detected on T1- weighted MRI. (f) Diffusion-weighted MRI did not identify them. (g) Cerebral blood flow almost normalized 6 months after combined bypass surgery on both sides. (h) The “striated” hyperintensity lesions completely disappeared 6 months after surgery. (i) The “patchy” hyperintensity lesions regressed 6 months after surgery and further diminished 12 months after surgery(arrows)
Case 2
A 10-year-old girl complained of transient weakness of the right extremities and severe headache attacks. Neurological examinations on admission revealed no neurological deficits. Cerebral angiography showed severe stenosis of the terminal portion of the ICA on both sides. The perforating arteries were markedly dilated [
Figure 2:
Radiological findings of a 10-year-old girl with moyamoya disease (Case 2). (a) Right nternal carotid angiography demonstrated typical findings of moyamoya disease, including severe stenosis of the supraclinoid portion of the internal carotid artery and the horizontal portions of the anterior and middle cerebral arteries. (b) Left internal carotid angiography also showed very similar findings. Note the markedly dilated perforating arteries on both sides. (c) Single photon emission computed tomography (SPECT) demonstrated cerebral blood flow reduction in the frontal lobe on both sides. (d)Fluid-attenuated inversion recovery (FLAIR) image showed the “patchy” hyperintensity lesions (arrows) in the subcortical white matter and also the “striated” hyperintensity lesions (arrowheads) in the periventricular white matter. The “striated” lesions appear to radiate from the lateral ventricles. (e) Cerebral blood flow almost normalized 5 months after combined bypass surgery on both sides. (f) The “striated” hyperintensity lesions completely disappeared 5 months after surgery. The “patchy” hyperintensity lesions disappeared in the frontal pole on both sides 5 months after surgery, but was still observed in the left frontal subcortex (arrow). (g) The lesion completely disappeared 12 months after surgery
Case 3
A 22-year-old male suddenly developed visual field disturbance and numbness in the right arm. Neurological examinations on admission revealed the right homonymous hemianopsia and sensory disturbance of the right extremities. Magnetic resonance angiography showed severe stenosis of the terminal portion of the bilateral ICA and the left posterior cerebral artery [
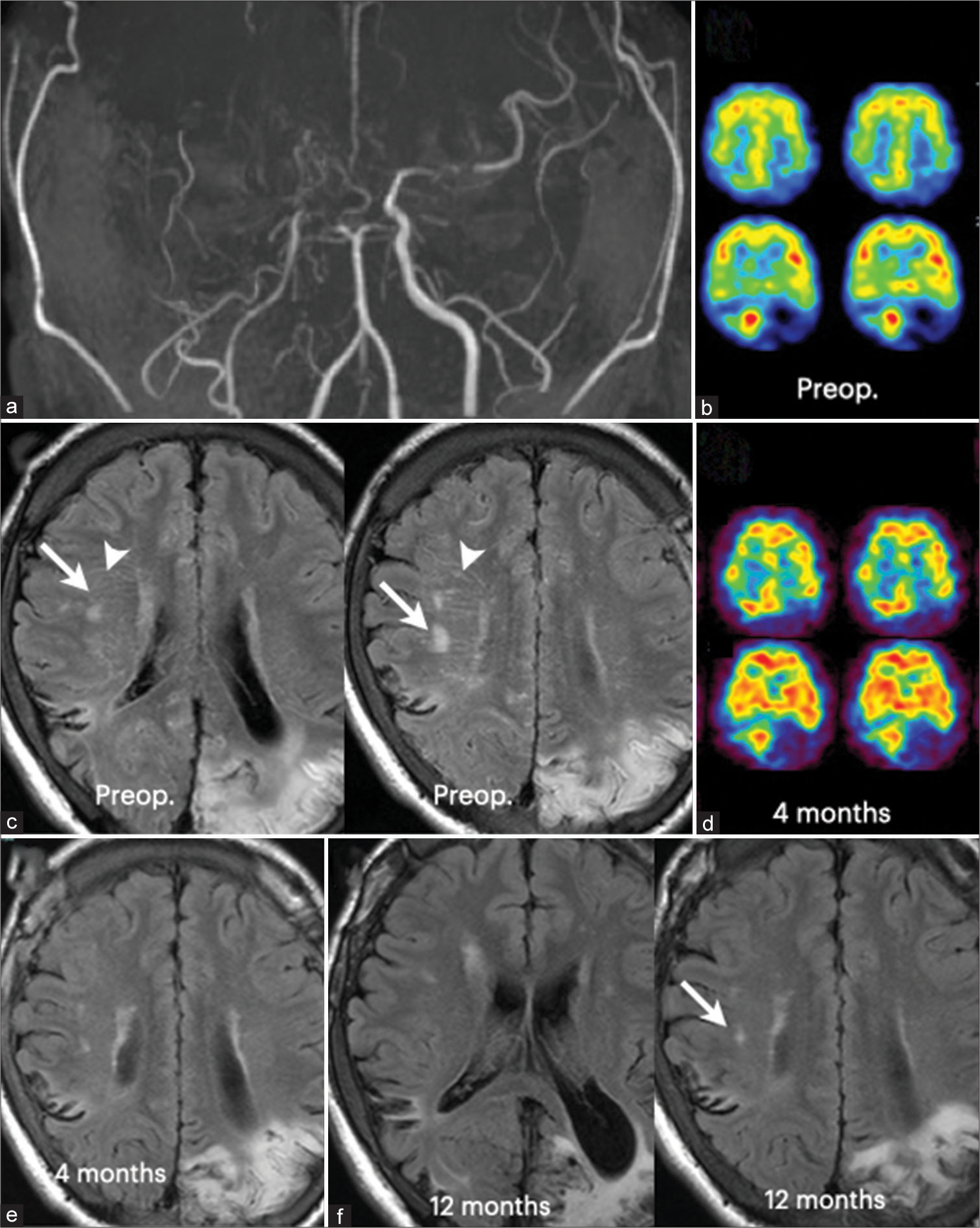
Figure 3:
Radiological findings of a 22-year-old male with moyamoya disease (Case 3). (a) MR angiography demonstrated typical findings of moyamoya disease, including severe stenosis of the supraclinoid portion of the internal carotid artery on both sides. (b) Single photon emission computed tomography (SPECT) demonstrated cerebral blood flow reduction in the cerebral hemispheres on both sides. (c) Fluid-attenuated inversion recovery (FLAIR) image showed the “patchy” hyperintensity lesions (arrows) in the right periventricular white matter and also the “striated” hyperintensity lesions (arrowheads) in the right periventricular white matter. The “striated” lesions appear to radiate from the right lateral ventricles. Note cerebral infarction in the left occipital lobe.(d) Cerebral blood flow almost normalized 4 months after combined bypass surgery on both sides, except for the left occipital lobe. (e) The “striated” hyperintensity lesions completely disappeared 4 months after surgery. The “patchy” hyperintensity lesions regressed 4 months after surgery. (f) But, they were still observed in the right side 12 months after surgery (arrow).
DISCUSSION
All three moyamoya patients presented here had a severe CBF reduction in the ICA territory, which resulted in a loss of hyperfrontality characteristic of children and young adults. FLAIR images demonstrated the hyperintensity lesions in the subcortical and/or periventricular white matter in five of six involved hemispheres. Interestingly, the WHM lesions could be divided morphologically into two patterns: the “patchy” and “striated” morphology, although there were no reports that denoted their morphology in the past. The “patchy” WMH lesions were found in the subcortical and/or periventricular white matter, while the “striated” WMH lesions were found only in the periventricular white matter. All three patients successfully underwent combined bypass surgery.[
As aforementioned, the WHM lesions in moyamoya patients may sometimes be reversible and disappear or diminish after surgical revascularization.[
The “striated” WMH seen in our cases are more prominent in the periventricular white matter than the subcortical region of the white matter, and they always coexist with the patchy WMH. Although the pathophysiology of “striated” WMH lesions is still unclear, these observations strongly suggest that the “striated” WMH lesions may develop under the same conditions as the “patchy” ones. When the white matter is exposed to persistent cerebral ischemia, demyelination and axonal degradation occur quickly in the ischemic core, and remyelination has been observed in the peri-ischemic area.[
In this report, surgical revascularization significantly improved cerebral hemodynamics. Then, the “striated” WMH lesions completely disappeared 4–6 months after surgery, while the “patchy” WMH lesions slowly diminished or disappeared over 12 months after surgery. The fact strongly suggests that chronic cerebral ischemia plays an important factor in to development of these WMH lesions. In addition, the “striated” WMH lesions and the “patchy” WMH lesions always coexist before surgery. These facts provide us with an important clue to explain the mechanisms underlying the development of WMH lesions in moyamoya disease. Thus, the WMH lesions may develop in two steps: the “striated” WMH lesions first develop along the axons due to cerebral ischemia, and then the “patchy” WMH lesions develop due to the further expansion of inflammation and vasogenic edema, probably provoked by more dense or more prolonged ischemia. The blood–brain barrier (BBB) breakdown may be related to the underlying mechanism. Therefore, the “patchy” lesions would be a more severe phenotype than the “striated” lesions.
CONCLUSION
We reported three cases of moyamoya disease with reversible WMH lesions, including two different morphologies: the “striated” and “patchy” patterns. Following surgical revascularization surgery, the former completely disappeared within six months, while the latter slowly regressed but did not disappear over 12 months. Based on their radiological findings and postoperative course, the “striated” WMH lesions may represent the inflammation or edema along the neuronal axons due to cerebral ischemia, while the “patchy” WMH lesions may represent vasogenic edema in the parenchyma through the BBB breakdown. Earlier diagnosis and effective surgical treatment may be important to resolve the WMH lesions on FLAIR images.
Ethical approval
The research/study was approved by the Institutional Review Board at Toyama University Hospital, number R2019057, dated August 30, 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
This study was supported by the Research Committee on Moyamoya Disease, sponsored by the Ministry of Health, Labor and Welfare of Japan (Grant No. 23FC101).
References
1. Ahtam B, Solti M, Doo JM, Feldman HA, Vyas R, Zhang F. Diffusion-weighted magnetic resonance imaging demonstrates white matter alterations in watershed regions in children with moyamoya without stroke or silent infarct. Pediatr Neurol. 2023. 143: 89-94
2. Calviere L, Ssi Yan Kai G, Catalaa I, Marlats F, Bonneville F, Larrue V. Executive dysfunction in adults with moyamoya disease is associated with increased diffusion in frontal white matter. J Neurol Neurosurg Psychiatry. 2012. 83: 591-3
3. Geraldo AF, Leitão C, Nunes J, Vila-Real M. Partially reversible confluent white matter lesions in a Caucasian child with moyamoya disease. Childs Nerv Syst. 2020. 36: 2605-8
4. Hara S, Hori M, Hagiwara A, Tsurushima Y, Tanaka Y, Maehara T. Myelin and Axonal damage in normal-appearing white matter in patients with moyamoya disease. AJNR Am J Neuroradiol. 2020. 41: 1618-24
5. Hara S, Hori M, Inaji M, Maehara T, Aoki S, Nariai T. Regression of white matter hyperintensity after indirect bypass surgery in a patient with moyamoya disease. Magn Reson Med Sci. 2019. 18: 247-8
6. Harada A, Fujii Y, Yoneoka Y, Takeuchi S, Tanaka R, Nakada T. High-field magnetic resonance imaging in patients with moyamoya disease. J Neurosurg. 2001. 94: 233-7
7. Jeong H, Kim J, Choi HS, Kim ES, Kim DS, Shim KW. Changes in integrity of normal-appearing white matter in patients with moyamoya disease: A diffusion tensor imaging study. AJNR Am J Neuroradiol. 2011. 32: 1893-8
8. Kazumata K, Tha KK, Narita H, Shichinohe H, Ito M, Uchino H. Investigating brain network characteristics interrupted by covert white matter injury in patients with moyamoya disease: Insights from graph theoretical analysis. World Neurosurg. 2016. 89: 654-65.e2
9. Komatsu K, Mikami T, Noshiro S, Miyata K, Wanibuchi M, Mikuni N. Reversibility of white matter hyperintensity by revascularization surgery in moyamoya disease. J Stroke Cerebrovasc Dis. 2016. 25: 1495-502
10. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
11. Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. 2010. 66: 1093-101 discussion 101
12. Kuroda S, Nakayama N, Yamamoto S, Kashiwazaki D, Uchino H, Saito H. Late (5-20 years) outcomes after STA-MCA anastomosis and encephalo-duro-myo-arteriopericranial synangiosis in patients with moyamoya disease. J Neurosurg. 2020. 134: 909-16
13. Li SJ, Xiong J, He Y, Xiao YY, Mao DA, Liu LQ. A rare case of pediatric moyamoya disease with reversible white matter lesions in a 3-year-old Chinese girl. Childs Nerv Syst. 2020. 36: 197-201
14. Nakamizo A, Kikkawa Y, Hiwatashi A, Matsushima T, Sasaki T. Executive function and diffusion in frontal white matter of adults with moyamoya disease. J Stroke Cerebrovasc Dis. 2014. 23: 457-61
15. Suzuki H, Mikami T, Kuribara T, Yoshifuji K, Komatsu K, Akiyama Y. Pathophysiological consideration of medullary streaks on FLAIR imaging in pediatric moyamoya disease. J Neurosurg Pediatr. 2017. 19: 560-6
16. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
17. Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A. Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res. 2003. 989: 172-9
18. Xu T, Feng Y, Wu W, Shen F, Ma X, Deng W. The predictive values of different small vessel disease scores on clinical outcomes in mild ICH patients. J Atheroscler Thromb. 2021. 28: 997-1008
19. Yang W, Jung KH, Kang DW, Lee EJ, Jeong HY, Chung M. Characteristics and clinical implication of white matter lesions in patients with adult moyamoya disease. Neurology. 2023. 100: e1912-21