- Department of Neurosurgery, University of Toyama, Toyama, Japan
- Department of Radiology, University of Toyama, Toyama, Japan
Correspondence Address:
Satoshi Kuroda, Department of Neurosurgery, University of Toyama, Toyama, Japan.
DOI:10.25259/SNI_571_2024
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Daina Kashiwazaki1, Shusuke Yamamoto1, Emiko Hori1, Naoki Akioka1, Kyo Noguchi2, Satoshi Kuroda1. Reversible sulcal fluid-attenuated inversion recovery hyperintensity after combined bypass surgery for moyamoya disease – A “crevasse” sign. 06-Sep-2024;15:322
How to cite this URL: Daina Kashiwazaki1, Shusuke Yamamoto1, Emiko Hori1, Naoki Akioka1, Kyo Noguchi2, Satoshi Kuroda1. Reversible sulcal fluid-attenuated inversion recovery hyperintensity after combined bypass surgery for moyamoya disease – A “crevasse” sign. 06-Sep-2024;15:322. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=13080
Abstract
Background: Transient fluid-attenuated inversion recovery (FLAIR) hyperintensity is often observed on the operated brain surface after direct or combined bypass surgery for moyamoya disease, but its pathophysiology and clinical significance are still obscure. This study was aimed to clarify the underlying mechanism and clinical significance.
Methods: This prospective study included 106 hemispheres of 61 patients with moyamoya disease and analyzed their radiological findings before and after combined bypass surgery. This study also included 11 patients who underwent superficial temporal artery to middle cerebral artery anastomosis for occlusive carotid artery diseases as the controls. Magnetic resonance imaging examination was serially repeated, and cerebral blood flow was measured before and after surgery. Signal intensity ratio (SIR) in the cortical sulci and cortex to the adjacent white matter on FLAIR images was calculated, and the postoperative SIR changes were semi-quantitatively evaluated to assess the temporal profile of postoperative FLAIR hyperintensity.
Results: Postoperative FLAIR hyperintensity occurred within the cortical sulci on the operated hemispheres in all moyamoya patients but not in patients with occlusive carotid artery diseases. SIR values started to increase immediately after surgery, peaked at about 4-fold at 4–13 days post-surgery, then declined, and recovered to baseline values over 28 days or later. The magnitude of this phenomenon was proportional to the severity of cerebral ischemia but not to postoperative hyperperfusion.
Conclusion: Reversible sulcal FLAIR hyperintensity specifically occurs in the operated hemispheres after direct bypass surgery for moyamoya disease. This “crevasse sign” may represent the mixture of the extensive leakage of oxygen and proteins from the pial arteries into the CSF.
Keywords: Bypass surgery, Cerebrospinal fluid, Cortical sulcus, Fluid-attenuated inversion recovery, Moyamoya disease, Oxygen
INTRODUCTION
Moyamoya disease is a unique cerebrovascular disease characterized by a progressive occlusion of the terminal portion of the internal carotid artery and its main branches. Moyamoya disease occurs in both children and adults and provokes a variety of cerebrovascular events, including transient ischemic attack, ischemic stroke, and hemorrhagic stroke.[
Based on these observations, this prospective study aimed to clarify the underlying mechanism and clinical significance of transient FLAIR hyperintensity after combined bypass surgery for moyamoya disease. For this purpose, we precisely specified the location of this phenomenon and semi-quantitatively evaluated the temporal and spatial profiles of this phenomenon. Furthermore, we assessed the relationship between this phenomenon and cerebral hemodynamics before and after surgery. The results would be valuable to understanding the underlying pathophysiology of this unique phenomenon in moyamoya disease to improve our perioperative management.
MATERIALS AND METHODS
Patients
This prospective study was approved by the Institutional Review Board of our hospital and was conducted according to the Declaration of Helsinki II. This study prospectively enrolled 61 patients with moyamoya disease and analyzed their radiological findings before and after surgical revascularization. All of them were diagnosed with moyamoya disease according to the diagnostic criteria set by the Research Committee on moyamoya Disease of Japan[
This study included 61 patients with moyamoya disease. There were 22 males and 39 females, including 21 children (6–18 years) and 40 adults. Mean age was 8.7 ± 4.3 years in children and 38.6 ± 8.7 years in adults. Surgical revascularization was performed on 106 hemispheres, including 37 hemispheres in children and 69 in adults. As the controls, this study also included 11 patients who underwent standard STA-MCA anastomosis for occlusive carotid artery disease. All of them were male, and their mean age was 71.1 ± 8.8 years.
Magnetic resonance imaging (MRI)
Using a 1.5-Tesla scanner (Magnetom Avanto, Siemens, Erlangen, Germany), all MRIs were obtained 3 days before surgery and were repeated 7 times after surgery. The timing of MR imaging was 0–3, 4–8, 9–13, 14–18, 18–22, 23–27, and 28 days after surgery. In addition to standard T1-, T2-, and diffusion-weighted images, the FLAIR image was acquired with the following parameters: TR/effective TE, 9,000/109; inversion time, 2500 ms; voxel size = 1.0 × 0.9 × 6.0 mm; FOV = 220 mm; parallel imaging mode = GRAPPA; accelerating factor = 2; and section thickness, 6 mm. Susceptibility-weighted image (SWI) was also acquired with the following parameters: TR/TE = 48/40 ms, FA = 15°; voxel size = 0.9 × 0.9 × 1.8 mm; FOV = 230 mm, parallel imaging mode = GRAPPA; accelerating factor = 3; and section thickness, 1.8 mm. Two certified neurosurgeons (DK and SK) who were blinded to clinical data independently evaluated the data and resolved disagreements by consensus.
SPECT
Before surgery, cerebral blood flow (CBF) before and after intravenous injection of 10-mg/kg acetazolamide (ACZ) was quantitatively measured with the 123I- N-isopropyl-piodoamphetamine injection and single-scan autoradiographic technique (GCA-9300/DI; Toshiba).[
CVR (%) = 100 × (CBFACZ –CBFrest)/CBFrest, where CBFrest and CBFACZ represent CBF before and after intravenous injection of ACZ, respectively. CBF was judged as reduced when the value was lower than 27 ml/min/100g, and CVR was judged as reduced when the value was lower than <14%. According to Kuroda’s classification, the hemispheres with normal CBF and reduced CVR were categorized into Type 2 ischemia, and those with reduced CBF and CVR were categorized into Type 3 ischemia.[
CBF measurement was repeated just after surgery and 2- and 7-days post-surgery. CBF was rated as abnormally elevated when the CBF in the operated MCA territory was higher than 150% of blood flow in the ipsilateral cerebellum.[
Bypass surgery
STA-MCA anastomosis and EDMAPS were performed on the hemispheres with the hemispheres with normal CBF but reduced CVR (Kuroda’s Type 2, n = 14) and those with reduced CBF and CVR (Kuroda’s Type 3, n = 92).[
The patients with atherosclerotic carotid artery diseases underwent STA-MCA single or double anastomosis onto the hemispheres with Kuroda’s Type 3 ischemia (n = 11).[
Semi-quantitative analysis of transient FLAIR hyperintensity
The temporal profile in the FLAIR intensity was semi-quantitatively analyzed using each patient’s set of FLAIR images. For this purpose, the 2.5-mm-diameter circular ROIs were placed on the cortex, adjacent white matter, and cortical sulci of each operated hemisphere at levels of the basal ganglia and the body of the lateral ventricle [
SIRCortex = Signal intensity cerebral cortex/Signal intensity white matter
SIRSulci = Signal intensity cortical Sulci/Signal intensity white matter
Then, the postoperative changes in SIR (ΔSIR) were calculated according to the following formula:
Δ SIRCortex = (SIRCortex postop.–SIRCortex preop.)/SIRCortex preop.
Δ SIRSulci = (SIRSulci postop.–SIRSulci preop.)/SIRSulci preop.
In these formulas, the white matter was selected as the internal reference because it is known that oxygen (O2) does not have no paramagnetic effects on the white matter.[
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Significant differences were set at P < 0.05. Variables were compared between the two groups using Mann–Whitney U-test.
RESULTS
Location and temporal profile
As shown in
Figure 1:
(a) Representative series of FLAIR images before and after bypass surgery on the right side for a 41-year-old female with moyamoya disease. In this case, typical “ivy” sign could be observed on the surface of parietal lobe (arrow). However, the “ivy” sign completely disappeared and postoperative FLAIR intensity was observed widely on the right hemispheres at 2 and 4 days after STA-MCA anastomosis and EDMAPS onto the right side (arrowheads). Postoperative FLAIR hyperintensity completely disappeared 28 days post-surgery. (b) Representative series of FLAIR images before and after bypass surgery on the right side for a 72-year-old male with internal carotid artery occlusion. Note that these findings were not found on the operated hemisphere after right STA-MCA anastomosis. FLAIR: Fluid attenuated inversion recovery, STA-MCA: Superficial temporal artery to middle cerebral artery, EDMAPS: Encephalo-duro-myo-arterio-pericranial synangiosis.
To specify the location of postoperative FLAIR hyperintensity in moyamoya disease, magnified MRI images of the brain surface were evaluated. Preoperative FLAIR images showed no abnormality on the brain surface [
Figure 2:
(a) Magnified FLAIR image before STA-MCA anastomosis and EDMAPS for a 35-year-old female with moyamoya disease. Note normal appearance before surgery. (b) Magnified FLAIR image at 2 days after surgery. Postoperative FLAIR hyperintensity was clearly observed within the cortical sulci at 2 days after STA-MCA anastomosis and EDMAPS (arrowheads). The cortical arteries within the cortical sulci are shown as low signal intensity because of flow void phenomenon on FLAIR image (arrow). (c) Magnified T2*-weighted MR at 2 days after surgery. The FLAIR hyperintensity was not detected as low signal intensity on T2*-weighted image, suggesting that this phenomenon is not due to postoperative blood accumulation within the cortical sulci (arrowheads). (d) A line graph shows the serial changes of signal intensity ratio (SIR) in the cortical sulci (open circle) and cortex (open triangle) in the MCA territory. In the cortical sulci, the SIR value started to increase within 3 days after STA-MCA anastomosis and EDMAPS, reaches the peak 4 to 13 days, and returns to the control level over 28 days post-surgery. However, the SIR values exhibited no significant changes throughout postoperative period in the cortex. **; P<0.01. FLAIR: Fluid attenuated inversion recovery, STAMCA: Superficial temporal artery to middle cerebral artery, EDMAPS: Encephalo-duro-myo-arteriopericranial synangiosis, SIR: Signal intensity ratio, POD: Postoperative day.
To confirm these results of visual inspection, the signal changes in the cortical sulci and cortex were semi-quantitatively analyzed.
Impacts of cerebral hemodynamics
To investigate the impact of the severity of cerebral ischemia on postoperative FLAIR hyperintensity, the ΔSIRSulci value was compared between Type 2 and Type 3 hemispheres. As shown in
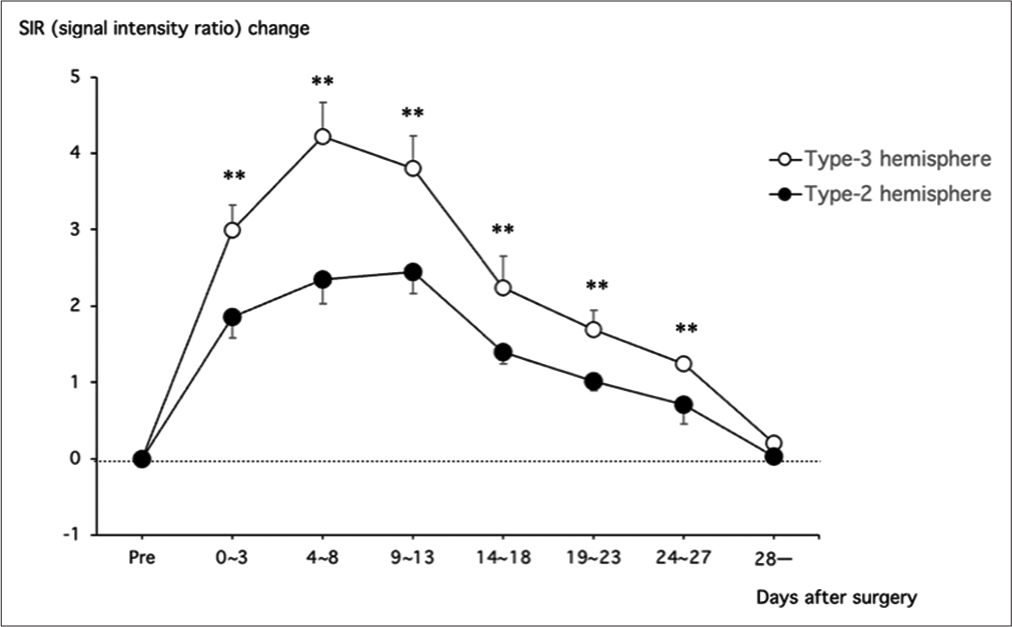
Figure 3:
A line graph illustrates the serial changes of signal intensity ratio (SIR) in the cortical sulci after superficial temporal artery to middle cerebral artery anastomosis and encephalo-duromyo-arterio-pericranial synangiosis for moyamoya hemispheres with Type 3 ischemia (open circle) and those with Type 2 ischemia (closed circle) in the middle cerebral artery territory. The SIR value was significantly higher in hemispheres with Type 3 ischemia than those with Type 2 ischemia within 28 days after surgery. **P < 0.01.
Type 2 hemispheres through 28 days after surgery (P < 0.01 at each time point). For example, the ΔSIRSulci value at 4–8 days post-surgery was 4.2 ± 0.4 and 2.4 ± 0.3 in hemispheres with Type 2 and Type 3 hemispheres, respectively (P < 0.01).
In a certain subgroup of moyamoya patients, postoperative FLAIR hyperintensity was observed in the ipsilateral occipital lobe outside the craniotomy. The ipsilateral posterior cerebral artery (PCA) was involved in a majority of them [
Figure 4:
(a, b) Representative preoperative MR angiography and postoperative FLAIR images at 7 days after right STA-MCA anastomosis and EDMAPS for a 42-year-old moyamoya patient without PCA involvement. Note that postoperative FLAIR hyperintensity was limited in the MCA area (arrowheads). (c, d) Representative preoperative MR angiography and postoperative FLAIR images at 7 days after right STA-MCA anastomosis and EDMAPS for a 48-year-old moyamoya patient with PCA involvement. Note that the finding was observed in the occipital lobe (d, arrows) as well as in the MCA area (d, arrowheads) in patient with PCA involvement (c, arrow). (e) A line graph demonstrates the serial changes of signal intensity ratio (SIR) in the cortical sulci of the occipital lobe after STA-MCA anastomosis and EDMAPS for moyamoya patients with PCA involvement (open circle) and those without (closed diamond). Note that the SIR changes in the occipital lobe were significantly pronounced in patients with PCA involvement than in those without. **; P<0.01. FLAIR: Fluid attenuated inversion recovery, STA-MCA: Superficial temporal artery to middle cerebral artery, EDMAPS: Encephalo-duro-myo-arterio-pericranial synangiosis,SIR: Signal intensity ratio, PCA: Posterior cerebral artery, POD: Postoperative day.
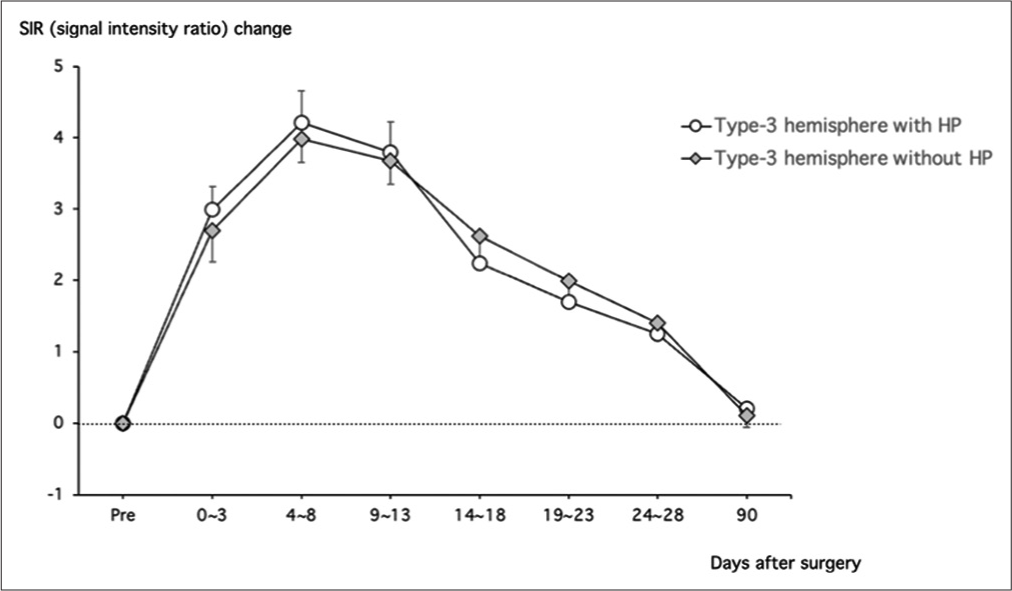
Postoperative hyperperfusion
Finally, we evaluated the impact of postoperative hyperperfusion on postoperative FLAIR hyperintensity in moyamoya disease. Postoperative hyperperfusion occurred in 37 (40.2%) of 92 Type 3 hemispheres. We compared ΔSIRSulci values between the hemispheres with postoperative hyperperfusion (n = 37) and those without (n = 55). The temporal profile was very similar between the two groups, and there were no significant differences in the ΔSIRSulci value at each time point. The peak Δ SIRSulci value at 4–8 days post-surgery was 4.2 ± 0.4 and 4.0 ± 0.3 in the hemispheres with hyperperfusion and those without, respectively [
Figure 5:
A line graph shows the serial changes of signal intensity ratio (SIR) in the cortical sulci after superficial temporal artery to middle cerebral artery anastomosis and encephalo-duro-myoarterio-pericranial synangiosis in the middle cerebral artery area of moyamoya patients with Type 3 ischemia. Note that there were no significant differences in the SIR changes between the patients with postoperative hyperperfusion (open circle) and those without (dashed diamond) after surgery. **P < 0.01.
DISCUSSION
This study mainly explores the following observations: First, postoperative FLAIR hyperintensity represents the increase in signal intensity within the cerebral sulci but not in the cortex or blood vessels on the brain surface. The finding strongly suggests that this phenomenon represents a postoperative change in the environment of CSF space.
Second, the phenomenon is observed in moyamoya patients but not in patients with occlusive carotid artery diseases, indicating that this “sulcal” FLAIR hyperintensity occurs through the pathophysiology specific to moyamoya disease. Third, the degree of postoperative “sulcal” FLAIR hyperintensity depends on the severity of cerebral ischemia before surgery, which strongly suggests that preoperative cerebral ischemia may play a key role in the occurrence of the phenomenon. On the other hand, postoperative hyperperfusion is not related to the occurrence of postoperative FLAIR hyperintensity.
Sulcal FLAIR hyperintensity has been recognized to occur through the failure to suppress the CSF signal on FLAIR imaging and previously described as “hyperintense CSF,” “leptomeningeal hyperintensity,” or “hyperintensity within the subarachnoid space.”[
First, sulcal FLAIR hyperintensity may occur through the leakage of protein from blood to CSF after surgery. Thus, vascular permeability may be increased by the blood-brain barrier (BBB) in moyamoya disease. For example, gadolinium contrast is known to leak from the pial arteries into the CSF due to the BBB breakdown in the acute stage of ischemic stroke.[
Second, FLAIR imaging is also known to be sensitive to the changes in O2 concentration in the CSF.[
Based on these observations, we speculate that postoperative FLAIR hyperintensity may develop through the combination of a sudden elevation of PCSFO2 and a protein leakage into the CSF after direct bypass procedures. Such drastic changes may continue for about 2 weeks, followed by a gradual resolution. Finally, we would like to propose giving the name “crevasse sign” to this uncommon radiological phenomenon that is specifically manifested in moyamoya disease because this phenomenon can be observed in the subarachnoid space of the cerebral sulci and is morphologically very similar to a series of “crevasse” deeply carved into a glacier.
CONCLUSION
Postoperative FLAIR hyperintensity specifically occurs in the subarachnoid space of the cerebral sulci on the operated hemispheres after direct bypass surgery for moyamoya disease. This “crevasse sign” may reflect an extensive leakage of O2 and proteins from the pial arteries into the CSF through an increased vascular permeability in the areas exposed to preoperative cerebral ischemia.
Ethical approval
The research/study was approved by the Institutional Review Board at Toyama University Hospital, number R2019057, dated August 30, 2019.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
This study was supported by the Research Committee on moyamoya Disease, sponsored by the Ministry of Health, Labor and Welfare of Japan (Grant No. 23FC1011).
References
1. Anzai Y. Superparamagnetic iron oxide nanoparticles: Nodal metastases and beyond. Top Magn Reson Imaging. 2004. 15: 103-11
2. Deliganis AV, Fisher DJ, Lam AM, Maravilla KR. Cerebrospinal fluid signal intensity increase on FLAIR MR images in patients under general anesthesia: The role of supplemental O2. Radiology. 2001. 218: 152-6
3. Elster AD, Moody DM. Early cerebral infarction: Gadopentetate dimeglumine enhancement. Radiology. 1990. 177: 627-32
4. Frigon C, Shaw DW, Heckbert SR, Weinberger E, Jardine DS. Supplemental oxygen causes increased signal intensity in subarachnoid cerebrospinal fluid on brain FLAIR MR images obtained in children during general anesthesia. Radiology. 2004. 233: 51-5
5. Hamano E, Kataoka H, Morita N, Maruyama D, Satow T, Iihara K. Clinical implications of the cortical hyperintensity belt sign in fluid-attenuated inversion recovery images after bypass surgery for moyamoya disease. J Neurosurg. 2017. 126: 1-7
6. Horie N, Morikawa M, Morofuji Y, Hiu T, Izumo T, Hayashi K. De novo ivy sign indicates postoperative hyperperfusion in moyamoya disease. Stroke. 2014. 45: 1488-91
7. Koenigsberg RA, Gul N, Faro S, Elfont R, Baker K, Tsai F. Hyperacute cerebral enhancement: The earliest predictor of hemorrhage by MR imaging?. J Neuroimaging. 1999. 9: 235-6
8. Kuroda S, Houkin K. Moyamoya disease: Current concepts and future perspectives. Lancet Neurol. 2008. 7: 1056-66
9. Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y. Novel bypass surgery for moyamoya disease using pericranial flap: Its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery. 2010. 66: 1093-101 discussion 101
10. Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: Can acetazolamide test predict it?. Stroke. 2001. 32: 2110-6
11. Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery. 1993. 32: 912-8 discussion 8-9
12. Kuroda S, Nakayama N, Yamamoto S, Kashiwazaki D, Uchino H, Saito H. Late (5-20 years) outcomes after STA-MCA anastomosis and encephalo-duro-myo-arteriopericranial synangiosis in patients with moyamoya disease. J Neurosurg. 2020. 134: 909-16
13. Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N. Cerebral oxygen metabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery disease. Stroke. 2006. 37: 393-8
14. Kuroda S, Shiga T, Ishikawa T, Houkin K, Narita T, Katoh C. Reduced blood flow and preserved vasoreactivity characterize oxygen hypometabolism due to incomplete infarction in occlusive carotid artery diseases. J Nucl Med. 2004. 45: 943-9
15. Kuroda S, Yamamoto S, Hori E, Kashiwazaki D, Noguchi K. Effects of superficial temporal artery to middle cerebral artery (STA-MCA) anastomosis on the cerebrospinal fluid gas tensions and pH in hemodynamically compromised patients. Acta Neurochir (Wien). 2023. 165: 3709-15
16. Kuroda S, Yamamoto S, Hori E, Kashiwazaki D, Noguchi K. Intraoperative monitoring of cerebrospinal fluid gas tension and pH before and after surgical revascularization for moyamoya disease. Surg Neurol Int. 2024. 15: 158
17. Maas AI, Fleckenstein W, de Jong DA, van Santbrink H. Monitoring cerebral oxygenation: Experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir Suppl (Wien). 1993. 59: 50-7
18. Melhem ER, Jara H, Eustace S. Fluid-attenuated inversion recovery MR imaging: Identification of protein concentration thresholds for CSF hyperintensity. AJR Am J Roentgenol. 1997. 169: 859-62
19. Narducci A, Yasuyuki K, Onken J, Blecharz K, Vajkoczy P. In vivo demonstration of blood-brain barrier impairment in Moyamoya disease. Acta Neurochir (Wien). 2019. 161: 371-8
20. Noguchi K, Ogawa T, Inugami A, Toyoshima H, Sugawara S, Hatazawa J. Acute subarachnoid hemorrhage: MR imaging with fluid-attenuated inversion recovery pulse sequences. Radiology. 1995. 196: 773-7
21. Noguchi K, Seto H, Kamisaki Y, Tomizawa G, Toyoshima S, Watanabe N. Comparison of fluid-attenuated inversion-recovery MR imaging with CT in a simulated model of acute subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2000. 21: 923-7
22. Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labour Sciences Research Grant for Research on Measures for Infractable Diseas. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo). 2012. 52: 245-66
23. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
24. Taoka T, Yuh WT, White ML, Quets JP, Maley JE, Ueda T. Sulcal hyperintensity on fluid-attenuated inversion recovery mr images in patients without apparent cerebrospinal fluid abnormality. AJR Am J Roentgenol. 2001. 176: 519-24
25. Uchino H, Kuroda S, Hirata K, Shiga T, Houkin K, Tamaki N. Predictors and clinical features of postoperative hyperperfusion after surgical revascularization for moyamoya disease: A serial single photon emission CT/positron emission tomography study. Stroke. 2012. 43: 2610-6
26. Zaharchuk G, Busse RF, Rosenthal G, Manley GT, Glenn OA, Dillon WP. Noninvasive oxygen partial pressure measurement of human body fluids in vivo using magnetic resonance imaging. Acad Radiol. 2006. 13: 1016-24