- Clinical Professor of Neurosurgery, Schoold of Medicine, State University of New York at Stony Brook, NY and ℅ Dr. Marc Agulnick,1122 Franklin Avenue Suite 106, Garden City, NY 11530.
Correspondence Address:
Nancy E Epstein, MD, FACS, Clinical Professor of Neurosurgery, School of Medicine, State University of New Yorrk at Stony Brook, NY and ℅ Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY 11530.
DOI:10.25259/SNI_566_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E Epstein. Review: Perspective on ocular toxicity of presurgical skin preparations utilizing Chlorhexidine Gluconate/Hibiclens/Chloraprep. 06-Jul-2021;12:335
How to cite this URL: Nancy E Epstein. Review: Perspective on ocular toxicity of presurgical skin preparations utilizing Chlorhexidine Gluconate/Hibiclens/Chloraprep. 06-Jul-2021;12:335. Available from: https://surgicalneurologyint.com/surgicalint-articles/10943/
Abstract
Background: Chlorhexidine Gluconate (CHG), Hibiclens (4% CHG with 4% Isopropyl Alcohol Detergent), and Chloraprep (i.e. labeled CHG-based solutions), utilized as preoperative surgical preparatory solutions may all cause severe oculotoxicity and ototoxicity. Alternatively, 10% Povidone-Iodine (PI) solutions without detergent demonstrate minimal toxic effects on the eyes and ears.
Methods: Based on studies from 1984 to 2021, we compared the safety/efficacy of CHG-based versus PI-based solutions utilized for presurgical skin preparation near the cornea/eyes and ears (i.e., predominantly for cranial or cervical spine surgery).
Results: Some studies documented that even minimal exposure (i.e., “splash risk”) during face/neck skin preparation with CHG-based solutions could result in irreversible corneal injury and ototoxicity. Within minutes to hours, CHG-based non-detergent solutions posed the risks of; corneal epithelial edema, anterior stromal edema, conjunctival chemosis, bullous keratopathy, and de-epithelialization. Notably, even occlusive dressings like Tegaderm could not protect against CHG penetration. Alternatively, PI-based solutions posed no to minimal ocular and/or ototoxicity, while often demonstrating comparable protection against surgical site infections (SSI).
Conclusion: Chlorhexidine Gluconate (CHG), Hibiclens, and Chloraprep (i.e. CHG-based solutions) are often used as skin preparations near the face/eyes/spine (i.e., particularly anterior/posterior cervical procedures). However, if these solutions come in contact with the eyes, corneal irritation, abrasions, and even blindness may result. Alternatively, PI non-detergent solutions demonstrate safety/minimal oculotoxicity/ototoxicity, while frequently showing comparable efficacy against SSI.
Keywords: Chloraprep, Corneal toxicity, Hibiclens, Oculotoxicity, Ototoxicity, Povidone-iodine solution, Skin preparation
INTRODUCTION
Chlorhexidine gluconate (CHG), Hibiclens (4% CHG and 4% Isopropyl Alcohol), and Chloraprep (i.e. CHG-based solutions) presurgical skin preparations have well-documented oculotoxicity and ototoxicity. Therefore, great care must be utilized to avoid eye and ear contact when utilizing these presurgical preparation solutions when performing cranial and/ or anterior or posterior cervical spine surgery, and occasionally, other procedures near the eyes/ears. Alternatively, Povidone-Iodine (PI) non-detergent solutions have demonstrated minimal eye/ear toxicity, while often showing comparable prophylaxis against surgical site infection (SSI). Here we reviewed the relative risks/benefits, and alternatives to CHG-based preoperative skin preparation solutions versus PI non-detergent solutions for patients undergoing procedures near the eyes/ears (i.e. cranial surgery, spinal surgery, and occasionally other procedures).
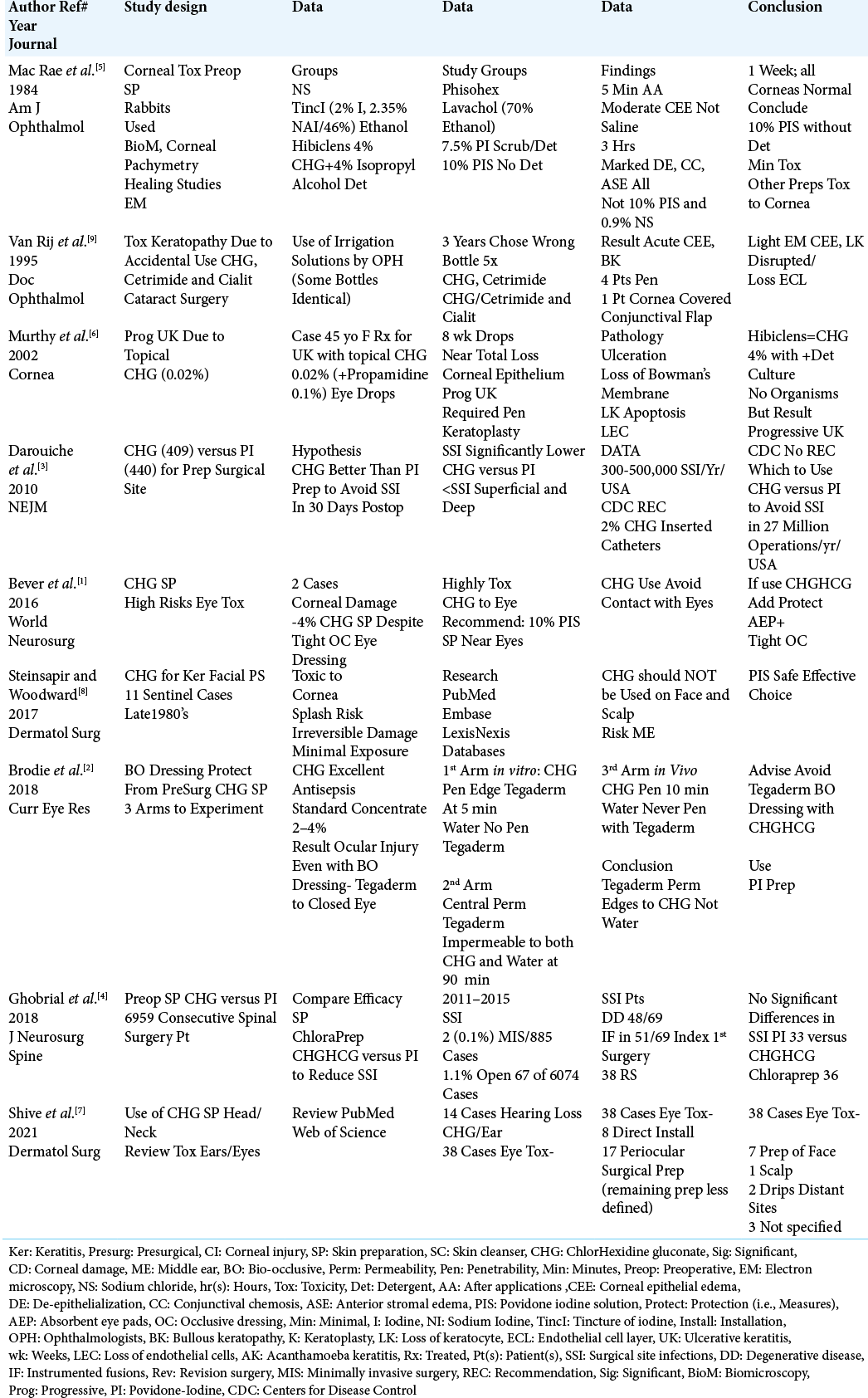
1984 RABBIT STUDY DOCUMENTED CORNEAL TOXICITY OF CHG/HIBICLENS
In 1984, Mac Rae et al. evaluated the corneal toxicity in rabbits of multiple skin preparations [
DOCUMENTATION OF CORNEAL TOXICITY FOR CHG-BASED SOLUTIONS
Multiple studies demonstrated significant corneal toxicity when using CHG-based preoperative skin preparation solutions for cranial, cataract, or spinal surgery [
STUDIES DOCUMENTING COMPARABLE OR SUPERIOR PREVENTION OF SSI UTILIZING CHG-BASED SOLUTIONS VERSUS PI SOLUTIONS FOR SURGICAL SKIN PREPARATIONS
Several studies documented that CHG-based versus PI-based skin preparation solutions provided comparable or superior prevention of SSI [
CORNEAL DAMAGE DESPITE UTILIZATION OF TIGHT AND/OR BIO OCCLUSIVE OCULAR DRESSINGS
Even tight or bio occlusive dressings (i.e. Tegaderm) did not adequately protect the eyes from dripping skin CHG-based preparations or “splashes” [
CONCLUSION
Multiple studies have documented the safety/efficacy of PI-based solution skin preparations when used near the eyes, ears, face, and neck (i.e., cranial, cervical spine, cataract/ surgery, other). Alternatively, CHG-based solutions (i.e., including Hibiclens and Chloraprep) have proven both oculotoxic and ototoxic. As both products have shown nearly comparable SSI prevention, careful attention must be given when using CHG over PI solutions near the eyes or ears.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Bever GJ, Brodie FL, Hwang DG. Corneal injury from presurgical chlorhexidine skin preparation. World Neurosurg. 2016. 96: 610.e1-4
2. Brodie F, Bever G, Hwang DG. Performance of bio-occlusive dressing as barrier protection from presurgical chlorhexidine skin preparation. Curr Eye Res. 2018. 5: 576-9
3. Darouiche RO, Wall MJ, Itani KM, Otterson MF, Webb AL, Carrick MM. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med. 2010. 362: 18-26
4. Ghobrial G, Wang MY, Green BA, Leyene HB, Manzano G, Vanni S. Preoperative skin antisepsis with chlorhexidine gluconate versus povidone-iodine: A prospective analysis of 6959 consecutive spinal surgery patients. J Neurosurg Spine. 2018. 28: 209-14
5. Mac Rae SM, Brown B, Edelhauser HF. The corneal toxicity of presurgical skin antiseptics. Am J Ophthalmol. 1984. 97: 221-32
6. Murthy S, Hawksworth NR, Cree I. Progressive ulcerative keratitis related to the use of topical chlorhexidine gluconate (0.02%). Cornea. 2002. 21: 237-9
7. Shive M, Hou Z, Zachary C, Cohen J, Rivers JK. The use of chlorhexidine as a skin preparation on the head and neck: A systematic review of ocular and ototoxicity. Dermatol Surg. 2021. 47: 34-7
8. Steinsapir KD, Woodward JA. Chlorhexidine keratitis: Safety of chlorhexidine as a facial antiseptic. Dermatol Surg. 2017. 43: 1-6
9. van Rij G, Beekhuis WH, Eggink CA, Geerards AJ, Remeijer L, Peis EL. Toxic keratopathy due to the accidental use of chlorhexidine, cetrimide and cialit. Doc Ophthalmol. 1995. 90: 7-14