- Clinical Professor of Neurosurgery, School of Medicine, State University of NY at Stony Brook, and c/o Dr. Marc Agulnick, 1122 Franklin Avenue Suite 106, Garden City, NY, 11530, USA.
Correspondence Address:
Nancy E. Epstein, MD, Clinical Professor of Neurosurgery, School of Medicine, State University of NY at Stony Brook, and c/o Dr. Marc Aglunick, 1122 Franklin Avenue Suite 106, Garden City, NY 11530, USA.
DOI:10.25259/SNI_153_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nancy E. Epstein. Review/Perspective On the Diagnosis and Surgical Management of Spinal Arachnoid Cysts. 25-Mar-2022;13:98
How to cite this URL: Nancy E. Epstein. Review/Perspective On the Diagnosis and Surgical Management of Spinal Arachnoid Cysts. 25-Mar-2022;13:98. Available from: https://surgicalneurologyint.com/surgicalint-articles/11492/
Abstract
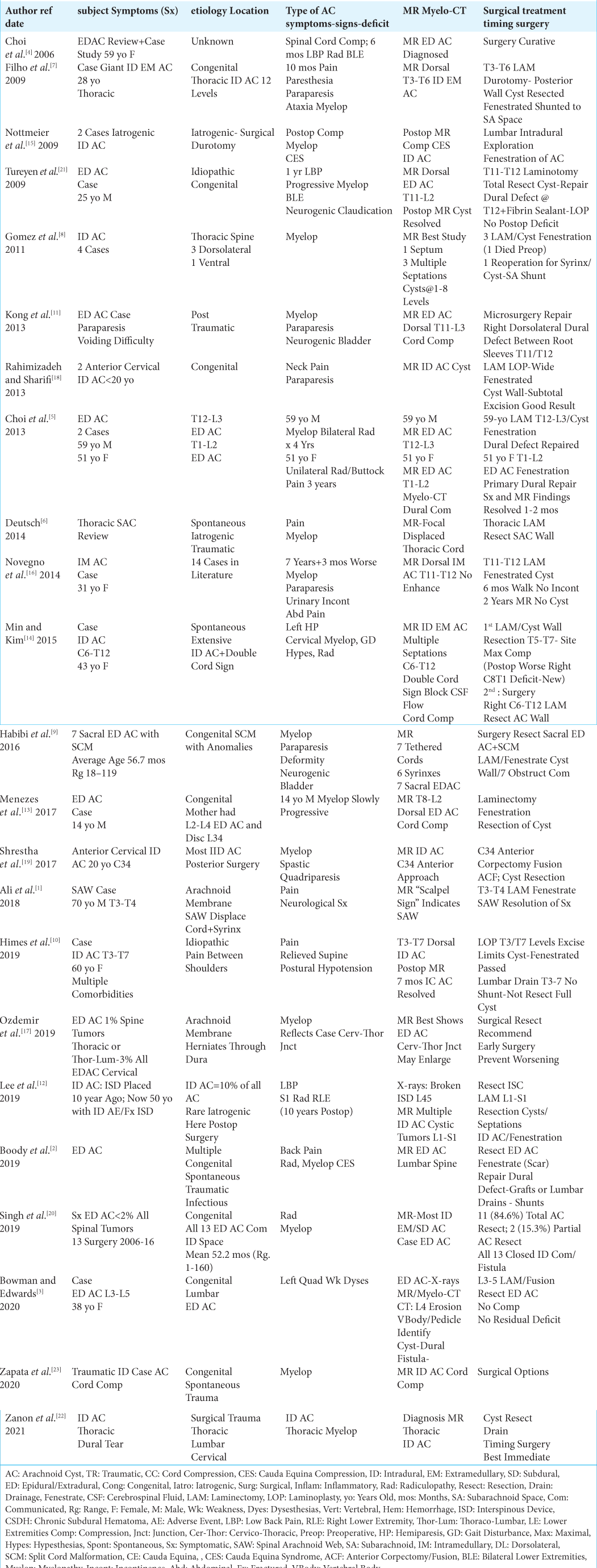
Background: Spinal arachnoid cysts (SAC) are typically congenital, spontaneous, traumatic (i.e., including iatrogenic/surgical), or inflammatory in origin. In descending order, they occur in the thoracic, lumbar, and cervical spine, and originate from focal entrapment of the arachnoid membrane. Arachnoid cysts represent 1–2% of all cystic spinal masses/tumors. The majority are extradural arachnoid cysts (EDAC) while 10% of all arachnoid cysts are intradural (IDAC) including subarachnoid, or extra-arachnoidal/subdural. Only rarely are they intramedullary in location. The clinical symptoms/signs of IDAC/EDAC include; intracranial hypotension (i.e., due to continued cerebrospinal fluid drainage), radiculopathy, and/or myelopathy.
Methods: Magnetic Resonance Images (MR) and Myelo-Computed Tomography (Myelo-CT) studies classically document the predominant dorsal location of IDAC/EDAC. They also show their extent and severity contributing to root, cord, and/or cauda equina compression. In the cervical/thoracic spine, MR/Myelo-CT studies classically show the “double cord” or “windsock” signs, while the “fake arachnoiditis sign” may be seen in the lumbar spine. The latter sign signals the presence of a circumferential extra-arachnoidal-subdural cyst that centrally “traps” the cauda equina. Note, that this resembles and is often misinterpreted as adhesive archnoiditis.
Results: Patients with significant SAC-related neurological deficits typically warrant early surgery. That surgery includes; partial/total resection/fenestration of cyst walls, and occlusion of communicating fistulas with or without accompanying shunts.
Conclusion: It is critical to recognize the clinical (i.e., intracranial hypotension, radiculopathy, and/or myelopathy) and radiographic MR/Myelo-CT signs (i.e., “double cord,” “windsock signs”, or “fake arachnoiditis sign”) of IDAC, EDAC, or intramedullary spinal arachnoid cysts to appropriately offer treatment. For those with significant neurological deficits, early surgery (i.e. optimally 0-
Keywords: Diagnosis/Surgery, Spinal Arachnoid Cysts, Intradural/Intramedullary, Intradural/Extramedullary, Extradural, Marsupialize, Spinal, Subdural Cyst, Cyst Resection, Double Cord/Windsock Signs, Fake Arachnoiditis Sign
INTRODUCTION
Spinal arachnoid cysts (SAC) may be congenital, spontaneous, traumatic (i.e. including iatrogenic/surgical), or due to inflammation [
EDAC AND IDAC VERSUS SPINAL SUBARACHNOID WEB (SAW)
MR followed by Myelo-CT studies document that the majority of IDAC or EDAC are dorsally located, resulting in severe nerve root, cervical/thoracic cord, and/or cauda equina compression [
Additionally, IDAC and EDAC must be differentiated from spinal arachnoid webs (i.e. SAW). These occur when abnormal arachnoid membranes within the subarachnoid space contribute to cord/root “displacement” and/or obstruction of CSF flow.[
INTRAOPERATIVE DURAL INJURY RESULTING IN POSTOPERATIVE IATROGENIC ARACHNOID CYSTS
Postoperative traumatic/iatrogenic arachnoid cysts are rare. Notably, estblishing the diagnosis of these lesions utilizing MR and/or Myelo-CT studies is critical so that they can be effectively treated early (i.e. optimally 0-< 24 hours after the onset of symptoms/signs), prior to the onset of a fixed neurological deficit [
ETIOLOGY OF INTRADURAL EXTRAMEDULLARY/SUBARACHNOID/ SUBDURAL ARACHNOID CYSTS (IDAC)
Intradural/extramedullary or subdural/extra-arachnoidal arachnoid cysts (IDAC) account for 10%[
Posterior surgical management of congenital IDAC
Two studies involving congenital IDAC warranted surgical resection, and fenestration; one patient required shunting to the subarachnoid space [
Anterior cervical corpectomy/fusion for managing a congenital IDAC
One study uniquely utilized a direct anterior cervical approach (i.e. anterior corpectomy/fusion ACF)) to resect a C3-C4 IDAC [
Posterior surgical management of spontaneous or traumatic (non-surgical) IDAC
Three studies, involving 6 patients were variously attributed to spontaneous or non-surgical/non-traumatic IDAC [
Posterior surgical management of traumatic surgical durotomies resulting in IDAC
In three studies, traumatic surgical durotomies resulted in IDAC requiring surgical resection, marsupialization, and cyst wall fenestration [
ETIOLOGY OF EDAC
EDAC are rare, and may be due to a congenital anomaly, appear spontaneously, or occur secondary to infection [
Posterior surgical management of presumed post-traumatic EDAC
EDAC comprise 1% of all spinal tumors, and are typically found at the thoracic/thoraco-lumbar regions, but only rarely (3%) occur in the cervical spine [
Posterior surgical management of congenital EDAC
Congenital EDAC typically appear in younger patients, and may occur in conjunction with other vertebral anomalies [
INTRAMEDULLARY SPINAL ARACHNOID CYST (IMAC)
Only very rarely are intramedullary SAC reported.[
DIFFERENTIATING SAW FROM IMAC, IDAC, AND EDAC
SAW may be traumatic or atraumatic in origin, and are due to the formation of abnormal arachnoid membranes within the subarachnoid space [
SHUNTING OPTIONS FOR IDAC, EDAC, AND IMAC
Several articles mention placing different types of shunts for IDAC and EDAC [
CONCLUSION
IDAC, EDAC, and IMAC occur due to outpouchings/entrapment of the arachnoid membrane fed by one-way fistulous tracts between the subarachnoid space and these cysts [
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Commentary
Preventing Arachnoid Cyst Recurrence
Intradural spinal arachnoid cysts may be dormant for many years and go unnoticed until gait difficulties and other signs of cord compression and myelopathy develop. At some point CSF dynamics change and the cyst becomes larger and pressurized. At that point you have a typically slowly growing mass in a confined space, and the first thing that gets compressed due to the pressure differential is the spinal cord. Once you suspect cord compression the workup and treatment need to expedited. MRI is usually the first choice of diagnostic studies given that it is noninvasive. But if MRI does not show you the top and bottom of the cyst clearly or the cyst is multiloculated, strong consideration should be given for obtaining a myelogram with CT. If there is delayed filling of the cyst post-myelogram, you potentially may need a delayed CT also.
It is important to look for such loculations that could make your fenestration surgery less effective. If there is a simple single cyst, knowing the top and bottom of the cyst can enable the surgeon to do fewer laminectomies by approaching the cyst at each end for fenestration, and resection of the cyst. Generous resection and removal of the cyst along the lateral wall away from the cord is safest, and still gives a low recurrence rate. You should be able to irrigate between the top and the bottom of the cyst resection. Passing a catheter between sites may break up loculations within the cyst, but will also yield a higher risk of cyst recurrence, and risk potential cord injury (i.e. depending on the type of catheter that is passed, and the number of passes needed to open the cyst fully). If you suspect the cyst is still loculated, it is recommended to perform additional levels of laminectomy to allow for better exposure, and a more thorough cyst resection. A watertight dural closure is essential. Neurologically patients typically have good outcomes if surgery is done early in the course of the cord compression. [
VENTRAL INTRADURAL CYSTS
Ventral Intradural cysts are thought to occur by a different mechanism and with resection, arachnoid elements are typically lacking. These cysts are thought to be formed from ventral dural defects that dissect the dura. These may also be associated with ventral cord herniation and are much harder to safely repair.[
EXTRADURAL ARACHNOID CYSTS
Extradural spinal arachnoid cysts can also cause cord compression and typically require multilevel laminectomy to expose the extent of the cyst to determine the site(s) of CSF communication so that the dural defect can be secured and occluded to minimize the chance of cyst recurrence.
CONCLUSION
Although uncommon, treatment with resection or fenestration of the arachnoid cyst (both IDAC and EDAC) offer a good prognosis with a low recurrence rate.[
Jamie Baisden, M.D
Department of Neurological Surgery, Professor of Neurological Surgery, Medical College of Wisconsin, Fellow of the American College of Surgeons(FACS), and Fellow of the American Association of Neurological Surgeons (FAANS)
E-mail: jbaisden@mcw.edu
References
1. Ali BH, Hamilton P, Zygmunt S, Yakoub . Spinal arachnoid web-a review article. J Spine Surg. 2018. 4: 446-50
2. Boody B, Lucasti CJ, Schroeder GD, Heller JE, Vaccaro AR. Extradural arachnoid cyst excision. Clin Spine Surg. 2019. 32: E403-6
3. Bowman JJ, Edwards CC. Extradural arachnoid cyst with bony erosion: A rare case report. J Spine Surg. 2020. 6: 736-42
4. Choi JY, Kim SH, Lee WS, Sung KH. Spinal extradural arachnoid cyst. Acta Neurochir (Wien). 2006. 148: 579-85
5. Choi SW, Seong HY, Roh SW. Spinal extradural arachnoid cyst. J Korean Neurosurg Soc. 2013. 54: 355-8
6. Deutsch H. Thoracic arachnoid cyst resection. Neurosurg Focus. 2014. 37:
7. Filho SC, da Silva HB, de Albuquerque LA, de Almeida JP, Santos FP, Sciubba DM. Giant intradural extramedullary arachnoid cyst of the thoracic spine. J Clin Neurosci. 2009. 16: 1369-71
8. Gomez E, Quiles AM, Pedraza S. Spinal arachnoid cyst as an infrequent cause of spinal cord compression. Neuroradiol J. 2011. 24: 535-45
9. Habibi Z, Hanaei S, Nejat F. Sacral extradural arachnoid cyst in association with split cord malformation. Spine J. 2016. 16: 1109-15
10. Himes BT, Kerezoudis P, Rajjoub KR, Shepherd DS, Bydon M. Resection of an extensive thoracic arachnoid cyst via less-invasive targeted laminoplasties. Int J Neurosci. 2019. 129: 397-400
11. Kong WK, Cho KT, Hong SK. Spinal extradural arachnoid cyst: A case report. Korean J Spine. 2013. 10: 32-4
12. Lee HG, Kang MS, Na YC, Jin BH. Spinal intradural arachnoid cyst as a complication of insertion of an interspinous device. Br J Neurosurg. 2019. p. 1-5
13. Menezes AH, Hitchon PW, Blouhy BJ. Symptomatic spinal extradural arachnoid cyst with cord compression in a family: Case report. J Neurosurg Spine. 2017. 27: 341-5
14. Min WK, Kim JE. Extensive spinal intradural arachnoid cyst exhibiting a “double cord sign” on magnetic resonance imaging. J Orthop. 2015. 13: 110-4
15. Nottmeier EW, Wharen RE, Patel NP. Iatrogenic intradural spinal arachnoid cyst as a complication of lumbar spine surgery. J Neurosurg Spine. 2009. 11: 344-6
16. Novegno F, Umana G, Muro LC, Fraioli B, Fraioli MF. Spinal intramedullary arachnoid cyst: Case report and literature review. Spine J. 2014. 14: e9-15
17. Ozdemir M, Kavak RP, Gulgonuf N. Spinal extradural arachnoid cyst in cervicothoracic junction. Spinal Cord Ser Cases. 2019. 5: 45
18. Rahimizadeh A, Sharifi G. Anterior cervical arachnoid cyst. Asian Spine J. 2013. 7: 119-25
19. Shrestha P, Shrestha P, Deykota UP. Excision of an anterior intradural arachnoid cyst of the cervical spine through central corpectomy approach. Eur Spine J. 2017. 26: 154-7
20. Singh S, Bhaisora KS, Sardhara J, Kanti K, Attri G, Mehrotra A. Symptomatic extradural spinal arachnoid cyst: More than a simple herniated sac. J Craniovertebr Junction Spine. 2019. 10: 64-71
21. Tureyen K, Senol N, Sahin B, Karahan N. Spinal extradural arachnoid cyst. Spine J. 2009. 9: e10-5
22. Zanon BC, Kanas M, Joaquim MA, Martins DE, Waichenberg M, Astur N. Posttraumatic arachnoid cyst in the thoracic spine with medullary compression: Case report. Ver Bras Orthop (São Paolo). 2021. 56: 114-7
23. Zapata HD, Monsalvez EU, Arribas PJ, Carretero MD, Fernandez CS, Garcia AF. Spinal cord compression secondary to traumatic intradural arachnoid cyst. Neurocirugia (Astur: Engl Ed). 2020. 31: 195-200






Fernando martinez salas
Posted February 27, 2023, 9:11 pm
Exelente articulo. Quisiera compartir el caso de una paciente . Muchas gracias