- Department of Neurosurgery, Hospital Santa Marcelina, São Paulo, Brazil.
Correspondence Address:
Raphael Vicente Alves, Department of Neurosurgery, Hospital Santa Marcelina, São Paulo, Brazil.
DOI:10.25259/SNI_764_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Raphael Vicente Alves, Leonardo Corrêa Sousa, Juliana Passinho Azevedo Rodrigues, Kaito Alves Carvalho Laube. Revisiting Parkinson: After six decades, his triangle remains useful. 21-Oct-2022;13:483
How to cite this URL: Raphael Vicente Alves, Leonardo Corrêa Sousa, Juliana Passinho Azevedo Rodrigues, Kaito Alves Carvalho Laube. Revisiting Parkinson: After six decades, his triangle remains useful. 21-Oct-2022;13:483. Available from: https://surgicalneurologyint.com/surgicalint-articles/11942/
Abstract
Background: The welcome advent and subsequent development of interventional neuroradiology led to an important paradigm shift in the management of many cerebrovascular diseases. This paradigm shift is especially true for carotid cavernous fistula and, for some time now, endovascular techniques are the mainstay approach for these lesions. The neurosurgical intervention should be adopted when the endovascular treatment is not practicable.
Case Description: We present the surgical solution adopted to treat a patient with an indirect carotid cavernous fistula (CCF), with quickly progressive symptoms, in which it was not possible to treat using the currently standardized endovascular technique. A pretemporal craniotomy with peeling of the dura mater at the middle fossa and exposure of Parkinson’s triangle on the lateral wall of the cavernous sinus was performed. Fibrin glue was injected by puncture of the lateral wall of the cavernous sinus for direct thrombosis of this sinus and the superior ophthalmic vein.
Conclusion: In the now far 60s, Parkinson already treated patients with CCF effectively and elegantly through the lateral wall of the cavernous sinus. Revisiting techniques from the past, associating them with the supplies widely available today, can sometimes be the solution to some especially challenging cases that we face in our profession.
Keywords: Carotid cavernous fistula, Cavernous sinus, Chemosis, Fibrin glue, Parkinson’s triangle
INTRODUCTION
“I see the future repeat the past;
I see a museum of great novelties;
Time doesn’t stand still.” (Arnaldo Brandão and Cazuza [1988], Brazilian musicians)
Human brain surgery is undoubtedly a professional exercise that requires from neurosurgeon extreme skills and competences, rigorous and continuous study, and an emotional stability which allow correct and accurate decisions in moments of great stress. Such professional complexity is one of the forces that drive this medical specialty to develop continuously and in an accelerated way in search of increasingly effective and safe solutions for patients. In this way, neurosurgery has undergone revolutionary changes.
If the past decades of the 20th century were a period of reinvention of the specialty with the development and/or technological improvement of several techniques (e.g., imaging, stereotactic guidance, navigation, microscopy, hemostasis, endoscopy, radiosurgery, neuromodulation, endovascular techniques, and molecular medicine),[
An obvious consequence of this progressive technical and technological development of neurosurgery is the progressive abandonment of more invasive surgeries in favor of the adoption of minimally invasive and increasingly effective techniques. It seems indisputable that we are walking on a no return path in the direction of a neurosurgery minimally invasive and maximally resolving. The welcome advent and subsequent development of interventional neuroradiology led to an important paradigm shift in the management of many cerebrovascular diseases. This paradigm shift is especially true for carotid cavernous fistula (CCF) and, for some time now, endovascular techniques are the mainstay approach for these lesions.[
However, as already said by the famous American pugilist Mike Tyson, “everyone has a plan until they get punched in the mouth.” What to do when our initial therapeutic plans fall into the ring and the illness “opens count” due to progressive symptoms? What to do when the modern and already standardized less invasive treatment techniques are not available? In these moments, it is interesting to review all the imaging examinations, re-discuss the challenging case with the multidisciplinary team, and revisit the techniques that our forerunners so elegantly developed in the past, and pairing them with a bit of creativity is often the solution available to winning some challenging “combats.”
The neurosurgical intervention should be adopted when the endovascular treatment is not practicable. In the present paper, we present the surgical solution adopted to treat a patient with an indirect CCF, with quickly progressive symptoms, in which it was not possible to treat using the currently standardized endovascular technique.
CASE DESCRIPTION
A 61-year-old male patient is admitted to our emergency department due to a complaint of progressive worsening of the previous left ocular symptoms. He reported having been diagnosed with CCF nearly 2 months ago and being under outpatient follow-up at another neurosurgical department. However, in the past 4 days before hospital admission, the patient worsened from of the previous proptosis and chemosis, in addition to the onset of ipsilateral eye ache and diplopia when looking to the left. He denied history of trauma and emphasized the spontaneous onset of the condition. He reported a personal medical history of hypertension, myocardial infarction, nondialysis chronic kidney disease, and smoking.
Admission brain computed tomography angiography (CTA) demonstrated the left superior ophthalmic vein engorgement and proptosis, suggesting the diagnosis reported by the patient. Brain angiography confirmed the diagnosis and showed that it was an indirect CCF (Barrow type D) whose arterial supply was through multiple small-caliber branches from the cavernous segment of the left internal carotid artery (ICA) and distal branches of the left maxillary artery. The arteriovenous shunt in the left cavernous sinus (CS) drained retrogradely into the ipsilateral superior ophthalmic vein (which was enlarged). During angiography, venous access was attempted to the CS for the endovascular treatment of the fistula; however, the thrombosed inferior and superior petrosal sinuses prevented the endovascular staff from reaching the CS.
After multidisciplinary discussion, a new endovascular approach to the fistula through the angular vein was chosen. Unfortunately, the transition from the angular to the superior ophthalmic vein proved to be too small and angled, preventing navigation with the microcatheter. On the 1st day after this new treatment attempt, the patient evolved with progressive worsening of symptoms and onset of visual deficit. This left visual deficit rapidly progressed to monocular blindness.
We assumed that such an unfavorable evolution would be due to thrombosis (post catheterization) of the angular and facial veins, and secondary increase in ocular venous hypertension. Given the impossibility of endovascular treatment and the unfavorable evolution of the case, we chose to perform a pretemporal craniotomy with peeling of the dura mater at the middle fossa and exposure of Parkinson’s triangle on the lateral wall of the CS. After surgically exposing the CS and observing slight arterial bleeding in the middle fossa peeling, we performed a temporary clipping of the common carotid artery to decrease arterial flow in the CS. Immediately after cervical clipping, a change in the appearance of bleeding (coloration) along the lateral wall of the CS was observed. Puncture of the lateral wall of the CS was performed in the Parkinson’s triangle and fibrin glue was injected (Tisseel Lyo, Baxter AG, Vienna, Austria) for direct thrombosis of the CS and superior ophthalmic vein (about 3 ml of fibrin glue was injected). The temporary clip from the common carotid was then removed and no bleeding was observed through the lateral wall of the CS.
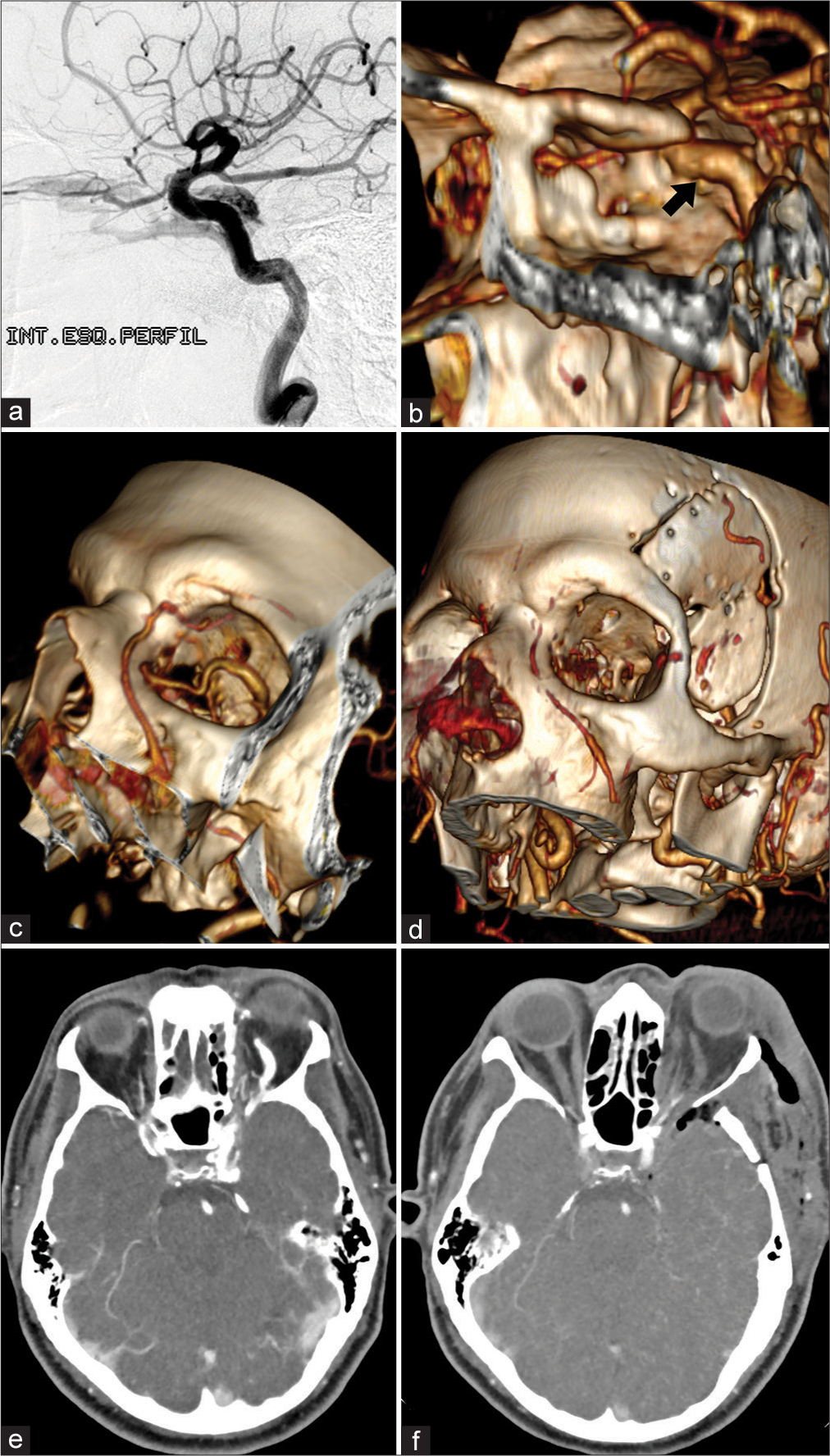
Postoperative CTA showed no surgical complications and no arterial filling of the CS or retrograde venous filling of the superior ophthalmic vein [
Figure 1:
Imaging exams. (a) Diagnostic brain angiography (left ICA – lateral view) showing a CCF. The arteriovenous shunt in the left CS drains retrogradely into the ipsilateral superior ophthalmic vein (which is enlarged). (b) Postoperative CTA reconstruction shows normal-appearing ICA (black arrow), preserved flow, and excludes arterial lesion or dissection. (c) Preoperative CTA reconstruction shows an important dilation of the superior ophthalmic vein inside the orbit. (d) Postoperative CTA reconstruction demonstrates the absence of contrast in the left superior ophthalmic vein and the absence of flow in the ipsilateral facial vein (which was well visualized in the preoperative imaging exam). We believe that the rapidly progressive worsening of visual function was secondary to angular and facial veins thrombosis after attempted catheterization of the superior ophthalmic vein. (e) Preoperative CTA with extravasation of contrast within the CS in the arterial phase and demonstrating the superior ophthalmic vein dilated and precociously contrasted. (f) Postoperative CTA with contrast exclusively in the cavernous segment of the ICA (no extravasation into the CS) and no contrast in the superior ophthalmic vein. ICA: Internal carotid artery, CS: Cavernous sinus, CTA: Computed tomography angiography, CCF: Carotid cavernous fistula.
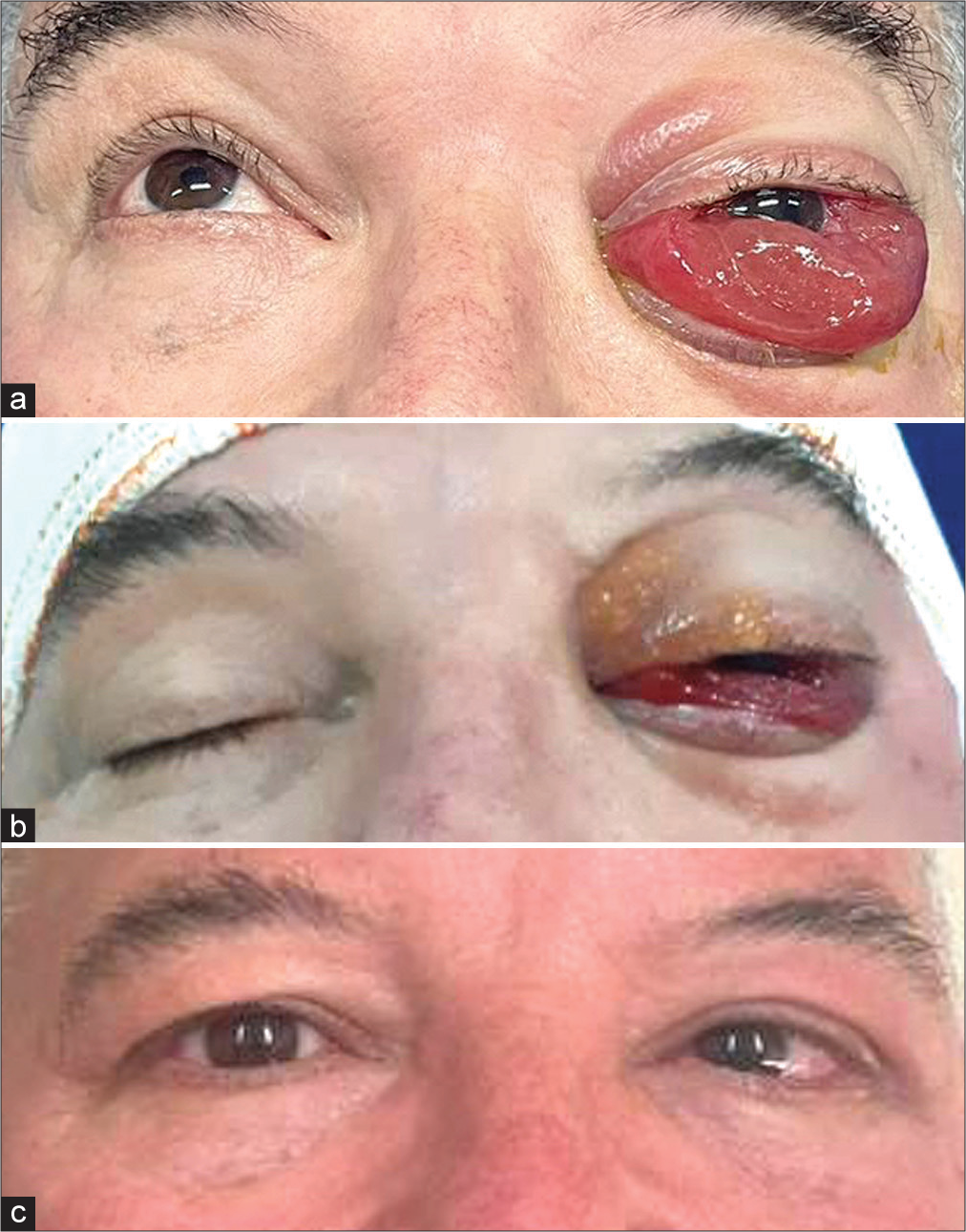
Figure 2:
(a) Patient on the operating table just before surgery. Amaurosis and paralysis of the cranial nerves responsible for extrinsic ocular movement (III, IV, and VI) of the left eye. Significant chemosis is noted. (b) Patient on the operating table immediately after surgery. Significant decrease in chemosis is observed. (c) At the outpatient consultation, 21 days after surgery, the patient had complete resolution of proptosis and chemosis, decreased of the VI left cranial nerve previous paresis, and unfortunately persistence of ipsilateral blindness.
DISCUSSION
According to Barrow et al.,[
It is well known that indirect fistulas have a relatively high incidence of spontaneous resolution or with conservative management due to their lower flow rate.[
The fact that indirect fistulas are generally associated with lower mortality and risk for intracranial complications emphasizes the importance of minimizing the morbidity and mortality of any therapeutic procedure.[
In general, due to rapid advances in techniques and devices, endovascular treatment is considered the primary treatment for CCFs and the transvenous embolization is the mainstay of treatment for indirect fistulas.[
Knowledge of the anatomy of the CS and the cavernous portion of the ICA is essential to an understanding of the etiology and treatment of CCF.[
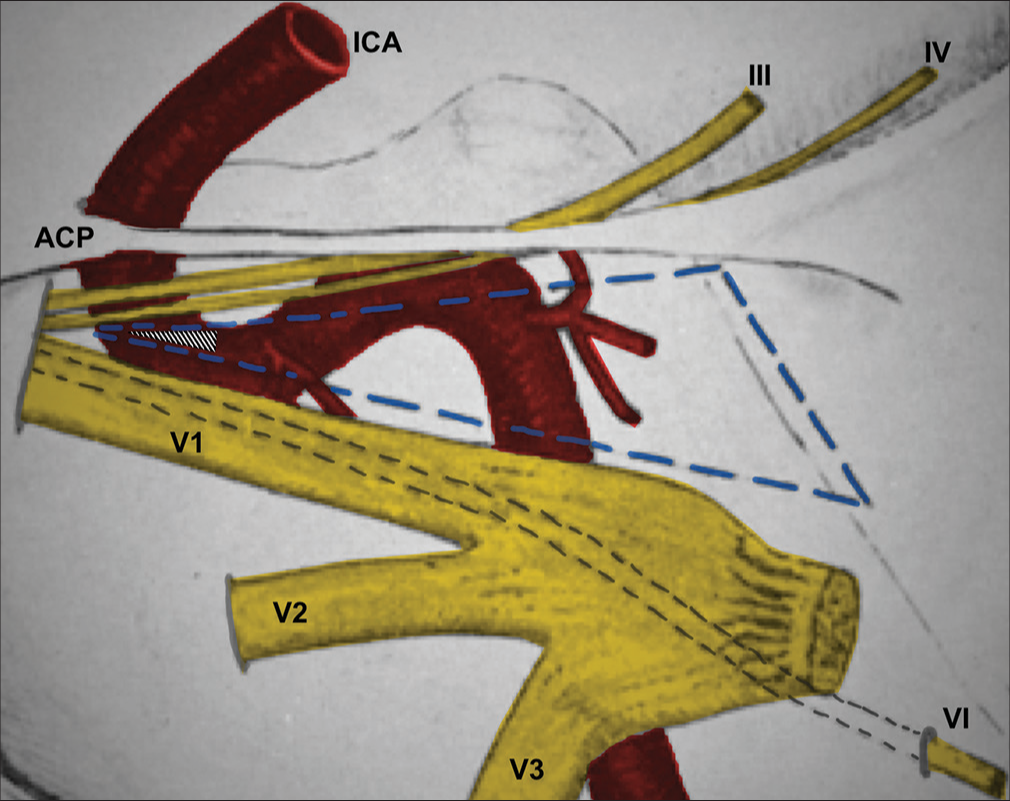
Figure 3:
Schematic drawing adapted and modified from Parkinson’s original publication.[
In the present case, our surgical strategy was: (i) dissect the common carotid artery and perform proximal cervical control to temporarily decrease arterial flow at the moment of fistula treatment; (ii) perform a pretemporal craniotomy and a microsurgical dural peeling of the middle fossa to expose the lateral wall of the CS; (iii) check the swollen CS with multiple points of small arterial bleeding on its side wall; (iv) identification of cranial nerves in the lateral wall of the CS for the identification of Parkinson’s triangle; (v) temporary clipping of the common carotid artery and verification of changes in the appearance and color of the bleeding by the middle fossa peeling; (vi) Parkinson’s triangle puncture and biological glue injection (initially at a higher speed [about 1 ml] and then at a lower speed [about 2 ml]); and (vii) removal of the temporary surgical clip from the common carotid artery and microscopically verified the total absence of bleeding through the lateral wall of the CS. Our surgery was entirely performed extradural and under microscopy. The patient evolved with clear and immediate improvement in proptosis, chemosis, and diplopia [
The CS has previously been considered a “no man’s land.” This was mainly due to the poor understanding of its surgical anatomy and the difficultness in establishing hemostasis within the CS.[
Fibrin glue is a two-component fibrin sealant made from pooled human plasma. The first component (sealant protein) is a highly concentrated fibrinogen solution, and the second component is a solution of thrombin, human albumin, and sodium chloride. It is applied with a dual-syringe delivery system with a single plastic tube that admixes the two components immediately before application by spraying or dripping onto the surgical target.[
The safety of fibrin glue injection into the CS for hemostasis is generally accepted and the venous flow has been shown to be re-established as early as 2–3 months postoperatively.[
CONCLUSION
In the now far 60s, Parkinson already treated patients with CCF effectively and elegantly through the lateral wall of the CS. His in-depth study of the anatomy of the CS is one of the most important bases that allowed a progressive construction of knowledge in the following decades. In the 1970s, Isamat was already using fibrin glue as an emboligenic agent for the treatment of CCF. Revisiting techniques from the past, associating them with supplies widely available today, can sometimes be the solution to some especially challenging cases that we face in our profession.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Andrade-Barazarte H, Chen Z, Feng C, Srinivasan VM, Furey CG, Lawton MT. Case report: Internal carotid artery thrombosis: A rare complication after fibrin glue injection for cavernous sinus hemostasis. Front Surg. 2021. 8: 730408
2. Apuzzo ML. The human cerebrum and the reinvention of neurosurgery. Neurosurgery. 2007. 61: 1
3. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985. 62: 248-56
4. Hasegawa H, Bitoh S, Obashi J, Maruno M. Closure of carotid-cavernous fistulae by use of a fibrin adhesive system. Surg Neurol. 1985. 24: 23-6
5. Hosobuchi Y. Electrothrombosis of carotid-cavernous fistula. J Neurosurg. 1975. 42: 76-85
6. Isamat F, Ferrer E, Twose J. Direct intracavernous obliteration of high-flow carotid-cavernous fistulas. J Neurosurg. 1986. 65: 770-5
7. Krayenbühl N, Hafez A, Hernesniemi JA, Krisht AF. Taming the cavernous sinus: Technique of hemostasis using fibrin glue. Neurosurgery. 2007. 61: E52 discussion E52
8. Krisht AF. Transcavernous approach to diseases of the anterior upper third of the posterior fossa. Neurosurg Focus. 2005. 19: E2
9. Lee JM, Park ES, Kwon SC. Endovascular management of cavernous sinus dural arteriovenous fistulas: Overall review and considerations. J Cerebrovasc Endovasc Neurosurg. 2021. 23: 293-303
10. Mullan S. Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg. 1979. 50: 131-44
11. Oishi H, Arai H, Sato K, Iizuka Y. Complications associated with transvenous embolisation of cavernous dural arteriovenous fistula. Acta Neurochir (Wien). 1999. 141: 1265-71
12. Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965. 23: 474-83
13. Parkinson D. Transcavernous repair of carotid cavernous fistula. Case report. J Neurosurg. 1967. 26: 420-4
14. Parkinson D. Carotid cavernous fistula: Direct repair with preservation of the carotid artery. Technical note. J Neurosurg. 1973. 38: 99-106
15. Parkinson D. Lateral sellar compartment: History and anatomy. J Craniofac Surg. 1995. 6: 55-68
16. Rhoton AL. The cavernous sinus, the cavernous venous plexus, and the carotid collar. Neurosurgery. 2002. 51: S375-410
17. Sekhar LN, Natarajan SK, Manning T, Bhagawati D. The use of fibrin glue to stop venous bleeding in the epidural space, vertebral venous plexus, and anterior cavernous sinus: Technical note. Neurosurgery. 2007. 61: E51 discussion E51
18. Texakalidis P, Tzoumas A, Xenos D, Rivet DJ, Reavey-Cantwell J. Carotid cavernous fistula (CCF) treatment approaches: A systematic literature review and meta-analysis of transarterial and transvenous embolization for direct and indirect CCFs. Clin Neurol Neurosurg. 2021. 204: 106601
19. Toyooka T, Otani N, Wada K, Tomiyama A, Ueno H, Fujii K. Effect of fibrin glue injection into the cavernous sinus for hemostasis during transcavernous surgery on the cerebral venous draining system. Oper Neurosurg (Hagerstown). 2017. 13: 224-31