- Department of Neurosurgery, University of Al-Nahrain, College of Medicine, Bagdad, Iraq,
- Department of Radiology, University of Al-Nahrain, College of Medicine, Bagdad, Iraq,
- Department of Neurosurgery, University of Baghdad, College of Medicine, Bagdad, Iraq,
- Department of Surgery, Al-Nahrain University, College of Medicine, Bagdad, Iraq,
- Department of Neurosurgery, University of Al-Mustansiriyah, College of Medicine, Bagdad, Iraq,
- Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, United States.
Correspondence Address:
Samer S. Hoz, Department of Neurosurgery, University of Cincinnati, Cincinnati, Ohio, United States.
DOI:10.25259/SNI_913_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Younus M. Al-Khazaali1, Noor A. Hummadi2, Mustafa Ismail3, Noor K. Al-Waely4, Fatimah O. Ahmed5, Samer S. Hoz6, Norberto Andaluz6. Right-sided aortic arch with complex anomalies presented with transient ischemic attack. 28-Oct-2022;13:493
How to cite this URL: Younus M. Al-Khazaali1, Noor A. Hummadi2, Mustafa Ismail3, Noor K. Al-Waely4, Fatimah O. Ahmed5, Samer S. Hoz6, Norberto Andaluz6. Right-sided aortic arch with complex anomalies presented with transient ischemic attack. 28-Oct-2022;13:493. Available from: https://surgicalneurologyint.com/surgicalint-articles/11962/
Abstract
Background: The right-sided aortic arch (RAA) is an uncommon anatomical anomaly found in
Case Description: A 52-year-old male, with a history of recurrent multiple TIAs, presented immediately after the onset of blurred vision and left-sided weakness. The initial diagnostic cerebral angiogram revealed a left CCA stenosis of
Conclusion: We presented an extremely rare case of RAA with ALSA associated with a group of extra rare anomalies. Understanding the anatomical variants of RAA and its characteristics is critical to improving the management and follow-up of patients with such anomalies.
Keywords: Aberrant left subclavian artery, Kommerell’s diverticulum, Pancake kidney, Right-sided aortic arch, Vascular ring
INTRODUCTION
The right-sided aortic arch (RAA) is an uncommon anatomical anomaly found in <0.1% of the adult population due to the continuation of the right fourth embryologic aortic arch and in-folding of the left aortic arch, with only half of the cases (0.05%) being associated with an aberrant left subclavian artery (ALSA).[
RAA often exhibits no symptoms and most adulthood diagnoses are unintentional. Dyspnea, coughing, and difficulty swallowing are the major symptoms that appear once Kommerell’s diverticulum compresses the surrounding structures.[
However, in this article, we report a case of RAA anomaly with an ALSA and Kommerell’s diverticulum associated with aneurysmal dilation of the ascending aorta, left common carotid artery (CCA) stenosis of approximately 30–40%, and pancake kidney presented with a transient ischemic attack (TIA). This is the first case in the literature that discusses such associations, especially in a symptomatic patient with neurological rather than tracheaesophageal symptoms with the absence of the steal phenomenon. Furthermore, our experience will provide a helpful report on the neuroendovascular aspect of diagnosing this condition.
CASE PRESENTATION
A 52-year-old male, with a history of recurrent multiple TIAs, presented immediately after the onset of blurred vision and right-sided weakness, which we confirmed with the physical examination. This patient has no medical history of TIA risk factors. Based on the clinical history and examination, a left internal carotid artery (ICA) stenosis was suspected and the patient was admitted for immediate therapeutic cerebral catheter angiography.
The initial diagnostic cerebral angiogram revealed a left CCA stenosis of <30%, with normal posterior circulation vasculature. However, we could not examine the anterior circulation of the brain due to technical difficulties, which we will discuss later. As a result, the therapeutic catheterization was discontinued and medical treatment was warranted. A computed tomography angiography (CTA) of the head, neck, chest, and abdomen was recommended for a more thorough examination.
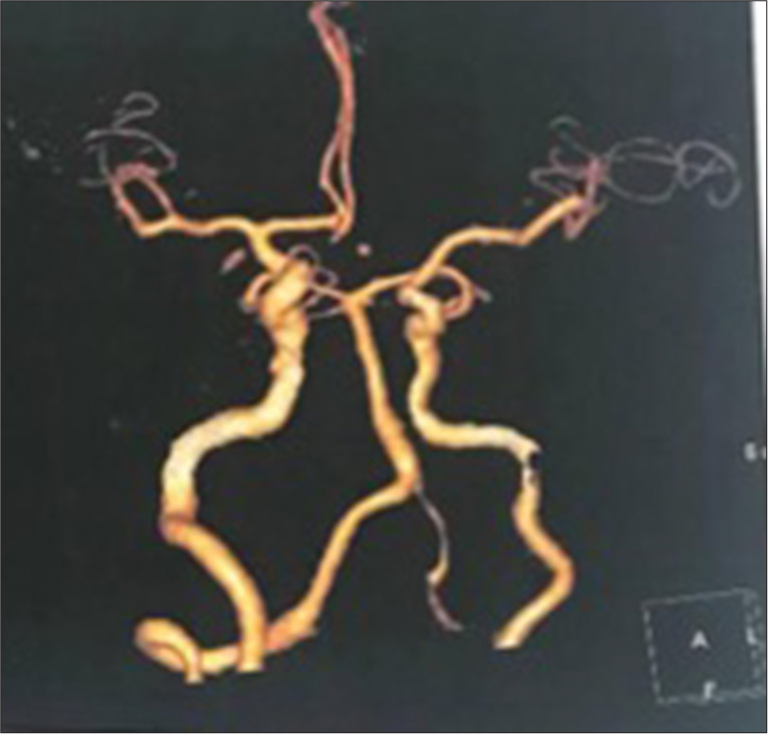
The native CT study of the brain after 45 days of catheterization showed signs of watershed infarction involving the right posterior frontal and parieto-occipital cortex. The CTA confirmed diffuse stenosis of nearly 30–50% in the left CCA and ICA, involving the entire vessel length, with diffuse circumferential mural thickening. However, a segment of mild narrowing (nearly 30%) was seen involving the right CCA measuring 30 mm in length, with a normal diameter of the right ICA. The circle of Willis was normal with a hypoplastic left A1 segment of the anterior cerebral artery with no vessel wall irregularities [
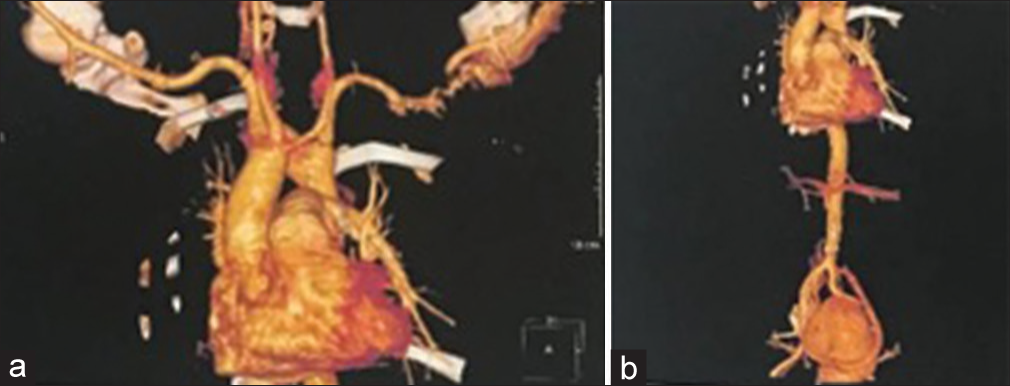
The diagnosis of RAA was made with CTA of the thoracic and abdominal aorta, which revealed Type 2 RAA, with ALSA, which had a bullous dilatation at its origin that suggests Kommerell’s diverticulum [
Another two unexpected findings on CTA were a persistent left-sided superior vena cava (PLSVC) that ended in the coronary sinus, then to the right atrium. A single pelvic fused renal mass (Pancake kidney) was discovered at the lower end plate of the L4 to S3 vertebrae, with a single ureter terminating into the left vesicoureteral junction. Moreover, a single renal artery arises from the right common iliac artery and divides later into two major arteries.
Figure 2:
(a) Computed tomography angiography (CTA) of the thoracic aorta (anteroposterior view) revealing the branches of the aortic arch in the following order: left common carotid artery, right common carotid artery, right subclavian artery, and finally, the aberrant left subclavian artery with aneurysmal origin (Kommerell’s diverticulum). (b) CTA of the abdominal aorta (anteroposterior view) shows the pancake kidney.
Technical difficulties during diagnostic cerebral catheterization
Highlighting the endovascular difficulties is critical since the atypical anatomy will affect the entire procedure, from increased operation time to failing to complete the examination. This case formulates in a retrograde fashion as we did not recognize the difficulty until after the catheterization had already begun.
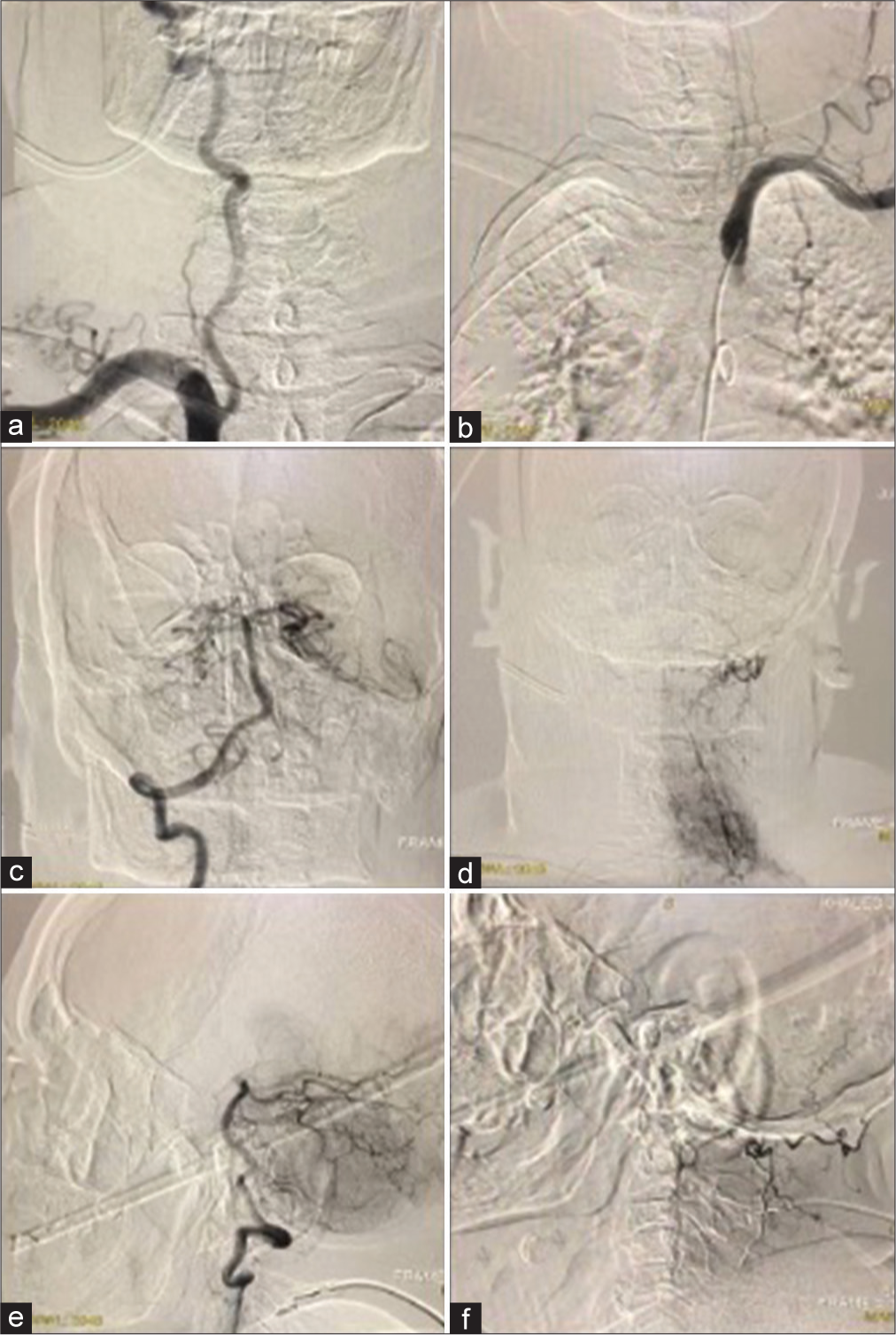
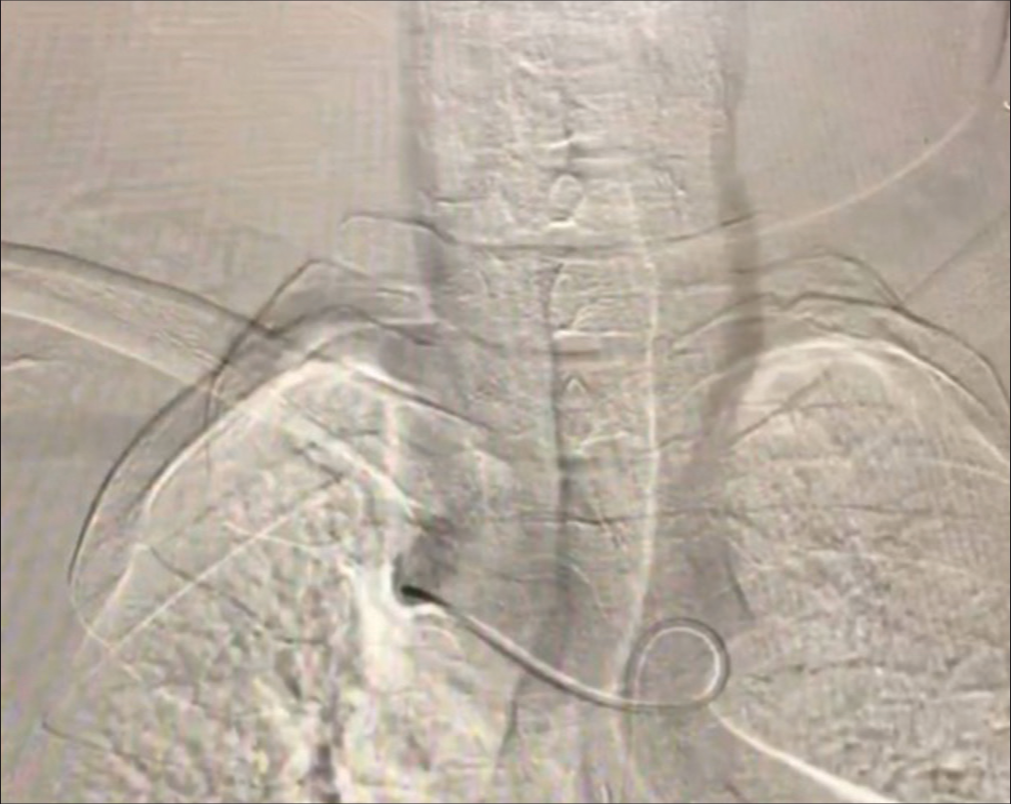
The cerebral angiography was performed using a 5-F (Vert; Merit) diagnostic catheter through a right trans-femoral route. Because the anatomical characteristics of a RAA limit catheter navigation, the first attempts to reach any of the main arteries of the Aortic Arch were unsuccessful. As a result, we replaced the (Vert; Merit) catheter with a (SIM1; Merit) catheter, which failed to reach them despite several maneuvers performed by the examiner. Finally, we shifted to the (SIM2; Merit) catheter, which yielded some results.
The (SIM2; Merit) catheter was placed into the aortic arch. First, we navigated the catheter at the left SCA origin; injection of the contrast material showed a small diameter left SCA and hypoplastic left VA. The origin of the left SCA was dilated, known as Kommerell’s diverticulum [
The carotids were the major challenge. We could not visualize their shadows by injecting contrast material near their origins or accessing them even with varying angles and maneuvers; all these efforts were unsuccessful in visualizing them. After several attempts of catheter navigation, the injection of the contrast material at the ascending aorta successfully visualized them only [
DISCUSSION
RAA is a rare anatomical abnormality established in <0.1% of the adult population when the aortic arch crosses the right main stem bronchus to the right of the trachea and esophagus and descends along the right side of the vertebral bodies.[
In addition, in Type 3 RAA, the left SCA is isolated and not connected to the aorta. However, because it is related to the pulmonary artery through the ductus arteriosus, it may also be associated with vertebrobasilar inefficiency and subclavian steal syndrome. The most frequent variety is Type 2, which accounts for approximately 40% of all RAA cases, while Type 3 is extremely uncommon.[
The RAA with ALSA is formed during embryonic development as a result of the continuation of the right fourth embryologic aortic arch and in-folding of the left aortic arch, with regression of the segment between the left CCA and LSCA so that the left fourth arch becomes the proximal SCA rather than the definitive aortic arch.[
Patients with a right aortic arch with a left ligamentum arteriosum are more likely to develop Kommerell’s diverticulum, which is a remnant part of the left fourth aortic arch arising as a left SCA aneurysm.[
In general, patients with Type II RAA are primarily asymptomatic. Unless the atherosclerotic changes of the anomalous vessels, dissection, and aneurysms develop, 5% of adult patients with aberrant SCA experience symptoms due to atherosclerotic changes. The morbidity caused by compression of adjacent structures results in dysphagia (dysphagia lusoria), dyspnea, stridor, wheezing, cough, recurrent pneumonia, obstructive emphysema, or chest pain by structure compression.[
We report the first case of Type 2 RAA to present with the left side anterior circulation TIA and to be accompanied by five different anomalies as the following:
Kommerell’s diverticulum Aneurysmal dilation of the ascending aorta Bilateral CCA and Left ICA stenosis PLSVC Pancake kidney
RAA is a quite rare variant of the aortic arch, not to mention that the association with ALSA is scarcer (0.05%), particularly when associated with Kommerell’s diverticulum.[
Based on a literature review of adult cases with Type 2 RAA, done between 2011 and 2019, two out of 31 cases presented anterior circulation abnormalities of nonatherosclerotic etiologies.[
Various factors can influence atherosclerotic changes. Aging might also play a role in influencing a variety of functional and structural alterations of the large arteries by a compound interaction with related agents, including genetics, disease, and environmental agents. Those functional and structural alterations of the aortic wall are among the variations of the proximal aorta associated with age, which will likely lead to functional and configurational alterations of the aortic arch itself.[
On the other hand, PLSVC and pancake kidney were exceptionally unexpected associations with RAA. Furthermore, it is considered to be one of the rarest instances. PLSVC is an uncommon variance among other vascular malformations and it mostly connects to the right atrium within the coronary sinus. PLSVC is accountable for venous drainage of the left part of the head, neck, and nearly 20% of the left arm. It is usually asymptomatic and incidental findings with a prevalence range between (0.2% and 3%). However, it is considered the most frequent inborn abnormality of the venous system in the chest.[
Pancake kidney is an exceptionally rare congenital abnormality with no exact prevalence ratios in the literature. This anomaly involves a full union of the medial parenchyma of both kidneys, no interposing septum, a specific collecting system for each kidney, and anteriorly positioned ureters that enter the bladder normally. However, our report described a pancake kidney case with only a single ureter drained into the bladder’s left vesicoureteral junction.[
Preoperative diagnosis of RAA will be crucial in avoiding significant neuroendovascular difficulties. Noninvasive echocardiography can provide an overall image of the aortic arch. Furthermore, magnetic resonance angiography and CTA afford accurate evaluation of the aortic arch, which can also aid in surgical or interventional planning. Doppler examination of the vessel must also be made to confirm the aberrant vessel.[
CONCLUSION
We presented an extremely rare case of RAA with ALSA associated with a group of extra rare anomalies. Taking into consideration the incidental diagnosis of these anomalies and their uncommon occurrence, it is tremendously important to understand the anatomical variants of RAA and its characteristics to improve the intraoperative recognition of these vascular anomalies, which may aid in better management and follow-up of these anomalies.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Ari ME, Doğan V, Özgür S, Ceylan Ö, Ertuğrul İ, Kayalı Ş. Persistent left superior vena cava accompanying congenital heart disease in children: Experience of a tertiary care center. Echocardiography. 2017. 34: 436-40
2. Azizova A, Onder O, Arslan S, Ardali S, Hazirolan T. Persistent left superior vena cava: Clinical importance and differential diagnoses. Insights Imaging. 2020. 11: 110
3. Barranhas AD, Indiani JM, Marchiori E, Dos Santos AA, Rochitte CE, Nacif MS. Atypical presentation of Kommerell’s diverticulum. Arq Brasil Cardiol. 2009. 93: e101-3
4. Batheeb NA, Habbab LM, Qattan NM. Symptomatic stenosis of left subclavian artery from Kommerell’s diverticulum. Asian Cardiovasc Thorac Ann. 2015. 23: 1068-71
5. Blackwelder H, Madueme P, Dadlani G, Ivsic T. Multi-modality assessment of the aortic arch branching and vascular rings. Prog Pediatr Cardiol. 2020. 58: 101268
6. Cinà CS, Althani H, Pasenau J, Abouzahr L. Kommerell’s diverticulum and right-sided aortic arch: A cohort study and review of the literature. J Vascu Surg. 2004. 39: 131-9
7. Hori D, Tanaka M, Yamaguchi A, Adachi H. Type A aortic dissection, right-sided aortic arch, and thoracic aortic aneurysm. Asian Cardiovasc Thorac Ann. 2009. 17: 640-2
8. Karthekeyan BR, Sundar S, Rao S, Vakamudi M. Management of a patient with Kommerrell’s aneurysm causing tracheal and esophageal compression. Indian J Anaesth. 2009. 53: 358-61
9. Mantri SS, Raju B, Jumah F, Rallo MS, Nagaraj A, Khandelwal P. Aortic arch anomalies, embryology and their relevance in neuro-interventional surgery and stroke: A review. Interv Neuroradiol. 2022. 28: 489-98
10. Matakas JD, Gold MM, Sterman J, Haramati LB, Allen MT, Labovitz D. Bovine arch and stroke laterality. J Am Heart Assoc. 2020. 9: e015390
11. Mittal S, Sharma A, Dixit S, Sharma M. Kommerell diverticulum with right-sided aortic arch with aberrant left subclavian artery. Indian J Vascu Endovascu Surg. 2020. 7: 435-7
12. Morosetti D, Di Stefano C, Mondillo M, Pensabene MC, De Corato L, Bizzaglia M. Right-sided aortic arch with mirror image branching and situs solitus: A case of a 79 years old woman. Radiol Case Rep. 2019. 14: 1246-51
13. Mubarak MY, Kamarul AT, Noordini MD. Right-sided aortic arch with aberrant left subclavian artery from Kommerell’s diverticulum. Iran J Radiol. 2011. 8: 103-6
14. Ohtani T, Yamazaki T, Ohtaki H, Nakata S, Sasaguchi N, Kato N. Carotid artery stenting in right-sided aortic arch: A case report. NMC Case Rep J. 2016. 3: 9-12
15. Pasquali M, Sciascia N, Liviano GD, Manna GL, Zompatori M. Pancake kidney: When it is not a problem. BJR Case Rep. 2018. 4: 20170117
16. Priya S, Thomas R, Nagpal P, Sharma A, Steigner M. Congenital anomalies of the aortic arch. Cardiovasc Diagn Ther. 2018. 8: S26-44
17. Redheuil A, Yu WC, Mousseaux E, Harouni AA, Kachenoura N, Wu CO. Age-related changes in aortic arch geometry: Relationship with proximal aortic function and left ventricular mass and remodeling. J Am Coll Cardiol. 2011. 58: 1262-70
18. Sakamoto S, Shibukawa M, Tani I, Araki O, Oki S, Kiura Y. Carotid artery stenting in a patient with right-sided aortic arch with an aberrant left subclavian artery. Acta Neurochir. 2011. 153: 2169-73
19. Stefańczyk L, Szymczyk K, Stefańczyk K, Polguj M. The presence of a right aortic arch associated with severe stenosis of the right common carotid artery and steal phenomenon. Ann Vasc Surg. 2015. 29: 1656.e13-7
20. Türkvatan A, Büyükbayraktar FG, Ölçer T, Cumhur T. Congenital anomalies of the aortic arch: Evaluation with the use of multidetector computed tomography. Korean J Radiol. 2009. 10: 176-84
21. Yang MH, Weng ZC, Weng YG, Chang HH. A right-sided aortic arch with Kommerell’s diverticulum of the aberrant left subclavian artery presenting with syncope. J Chin Med Assoc. 2009. 72: 275-7
22. Zhyvotovska A, Yusupov D, Abdul R, Chandrakumar H, Hartt A, Akter K. Right-sided aortic arch with aberrant left subclavian artery in a pregnant female: A case report and literature review. Am J Med Case Rep. 2020. 8: 143-7