- Department of Surgery, Section of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan.
- Medical student, Aga Khan University Medical College, Aga Khan University, Karachi, Pakistan.
- Department of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan.
- Medical student, Ziauddin Medical College, Karachi, Pakistan.
- Department of Neurosurgery, Northwest School of Medicine, Peshawar, Pakistan.
Correspondence Address:
Syed Ather Enam, Department of Surgery, Section of Neurosurgery, Aga Khan University Hospital, Karachi, Pakistan.
DOI:10.25259/SNI_135_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Muhammad Shakir1, Aly Hamza Khowaja2, Ahmed Altaf3, Aimen Tameezuddin4, Syed Sarmad Bukhari5, Syed Ather Enam1. Risk factors and predictors of intraoperative seizures during awake craniotomy: A systematic review and meta-analysis. 08-Jun-2023;14:195
How to cite this URL: Muhammad Shakir1, Aly Hamza Khowaja2, Ahmed Altaf3, Aimen Tameezuddin4, Syed Sarmad Bukhari5, Syed Ather Enam1. Risk factors and predictors of intraoperative seizures during awake craniotomy: A systematic review and meta-analysis. 08-Jun-2023;14:195. Available from: https://surgicalneurologyint.com/surgicalint-articles/risk-factors-and-predictors-of-intraoperative-seizures-during-awake-craniotomy-a-systematic-review-and-meta-analysis/
Abstract
Background: Awake craniotomy (AC) aims to minimize postoperative neurological complications while allowing maximum safe resection. Intraoperative seizures (IOSs) have been a reported complication during AC; however, literature delving into the predictors of IOS remains limited. Therefore, we planned a systematic review and meta-analysis of existing literature to explore predictors of IOS during AC.
Methods: From the inception until June 1, 2022, systematic searches of PubMed, Scopus, the Cochrane Library, CINAHL, and Cochrane’s Central Register of Controlled Trials were conducted to look for published studies reporting IOS predictors during AC.
Results: We found 83 different studies in total; included were six studies with a total of 1815 patients, and 8.4% of them experienced IOSs. The mean age of included patients was 45.3 years, and 38% of the sample was female. Glioma was the most common diagnosis among the patients. A pooled random effect odds ratio (OR) of frontal lobe lesions was 2.42 (95% confidence intervals [CI]: 1.10–5.33, P = 0.03). Those with a pre-existing history of seizures had an OR of 1.80 (95% CI: 1.13–2.87, P = 0.01), and patients on antiepileptic drugs (AEDs) had a pooled OR of 2.47 (95% CI: 1.59–3.85, P
Conclusion: Patients with lesions of the frontal lobe, a prior history of seizures, and patients on AEDs are at higher risk of IOSs. These factors should be taken into consideration during the patient’s preparation for an AC to avoid an intractable seizure and consequently a failed AC.
Keywords: Awake craniotomy, Intraoperative seizures, Meta-analysis, Systematic review
INTRODUCTION
The role of awake craniotomy (AC) in neurosurgery has gained much traction over the years due to a large body of evidence demonstrating its safety and efficacy in improving surgical outcomes for patients with a wide variety of pathologies, such as intra and extra-axial tumors, epilepsy, and even vascular neurosurgery.[
Historically, it has been practiced for millennia, as the treatment for epilepsy, as trepanation.[
The anesthetist’s approach varies depending on patient factors, and particular characteristics of the surgery such as type and length. Some ACs may be performed without sedating the patient in the “awake-awake-awake” method. A “conscious sedation” technique with monitored anesthesia care keeps the patient moderately sedated, to an extent where spontaneous breathing is maintained, reducing the incidence of effects such as hypotension, respiratory depression, and apnea. The anesthesia is then weaned off before cortical mapping and resumed before continuing with closure. On the other hand, a “sleep-awake sleep” technique uses an awake period of cortical mapping between two periods of complete sedation under general anesthesia (GA).[
Once the consciousness has been assessed by a designated member of the anesthesia team, cortical mapping begins with a reevaluation of higher mental function, specific to the marked brain area. The process of cortical mapping may extend over 3 hours and can be exhausting for the patient and care providers alike.[
The benefits of the AC technique in tumor resection have been greatly discussed, with AC surgeries having shorter hospital stays (4 days vs. 9 days), and less frequent postoperative deficits (7% vs. 23%) in matched procedures done under GA.[
There are a few notable perioperative complications associated with an AC. Postoperative effects such as new motor and language defects may be found in the early postoperative period.[
Intraoperative seizures (IOSs) remain of particular interest as they can result in AC failure and significant morbidity. They may affect the ability to carry out further cortical mapping and monitoring of the patient. In cases where seizures do not resolve or convert to status epilepticus, emergency conversion to craniotomy under GA may be required.[
The objective of our study is to explore factors associated with the development of IOS during awake craniotomies.
MATERIALS AND METHODS
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) standards were used as a guideline for conducting this study.[
Search strategy
From the inception until June 1, 2022, we conducted a search of published literature using PubMed, Scopus, CINAHL, the Cochrane library, to identify studies reporting the risk factors of IOS during AC. The Medical Subject Headings database was used to search for relevant phrases, and the following free text terms were ultimately chosen as keywords: “Risk factors” OR “predictors” and “Intraoperative” and “Seizures” OR “Epilepsy” and “Awake Craniotomy.“ Furthermore, a search for gray literature was also performed. The search was restricted to the English language; the publishing date; however, was not constrained. Pediatric focused literature and animal studies were filtered out. The detailed search strategies for each database can be found in the [supplementary file].
Study selection
There were case-control, cross-sectional, prospective, retrospective cohort, and randomized clinical trials (RCT) included in the study. For this analysis, only those studies were taken into consideration which were in English language, articles of sample size (n > 40), studies that reported human adult patients of age above 18, articles on AC inside the operating room, studies which reported IOS, and risk factors of IOS. We excluded publications other than English, articles on craniotomy under GA, research without assessments of IOS risk variables, case reports, review articles, editorials, letters to the editor, meeting abstracts, animal testing, in vivo/in vitro research, biological studies, publications without data extraction, and studies lacking full texts. Two authors of this study went through each article’s title and abstract to eliminate studies that did not meet the criteria for inclusion. Independent reviews of the full texts of the remaining publications determined which research should be included in this meta-analysis. Conflicts were settled by peer discussion and eventual consensus.
Data extraction and outcome measures
The extraction of data was conducted using the data extraction for complex meta-analysis guide.[
Quality assessment
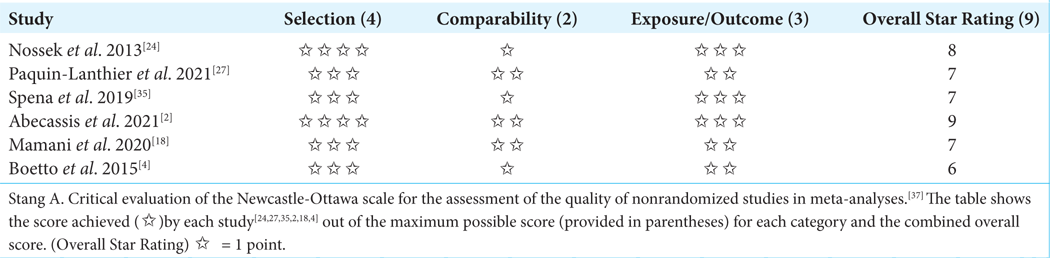
The Newcastle Ottawa quality assessment tool Newcastle-Ottawa scale (NOS) was used as reference to evaluate the collected papers’ quality.[
Statistical analysis
The statistical analysis of potential risk factors for IOS was conducted using the Cochrane Review Manager (RevMan 15). The mean difference (MD) reported with 95% confidence intervals (CI) was calculated for continuous variables. However, the OR with a 95% confidence level was used to analyze dichotomous variables. P < 0.05 was maintained as the level of significance for all analysis. Due to the diversity of the targeted population and length of included studies, a random effect model was used. In addition, when using meta-analysis to guide health-care decisions, the random-effects model is frequently favored. The I2 statistic and Cochran Q-test were used to assess heterogeneity. If the I2 statistic was >50% and the Q-test’s P < 0.10, heterogeneity was deemed significant.
RESULTS
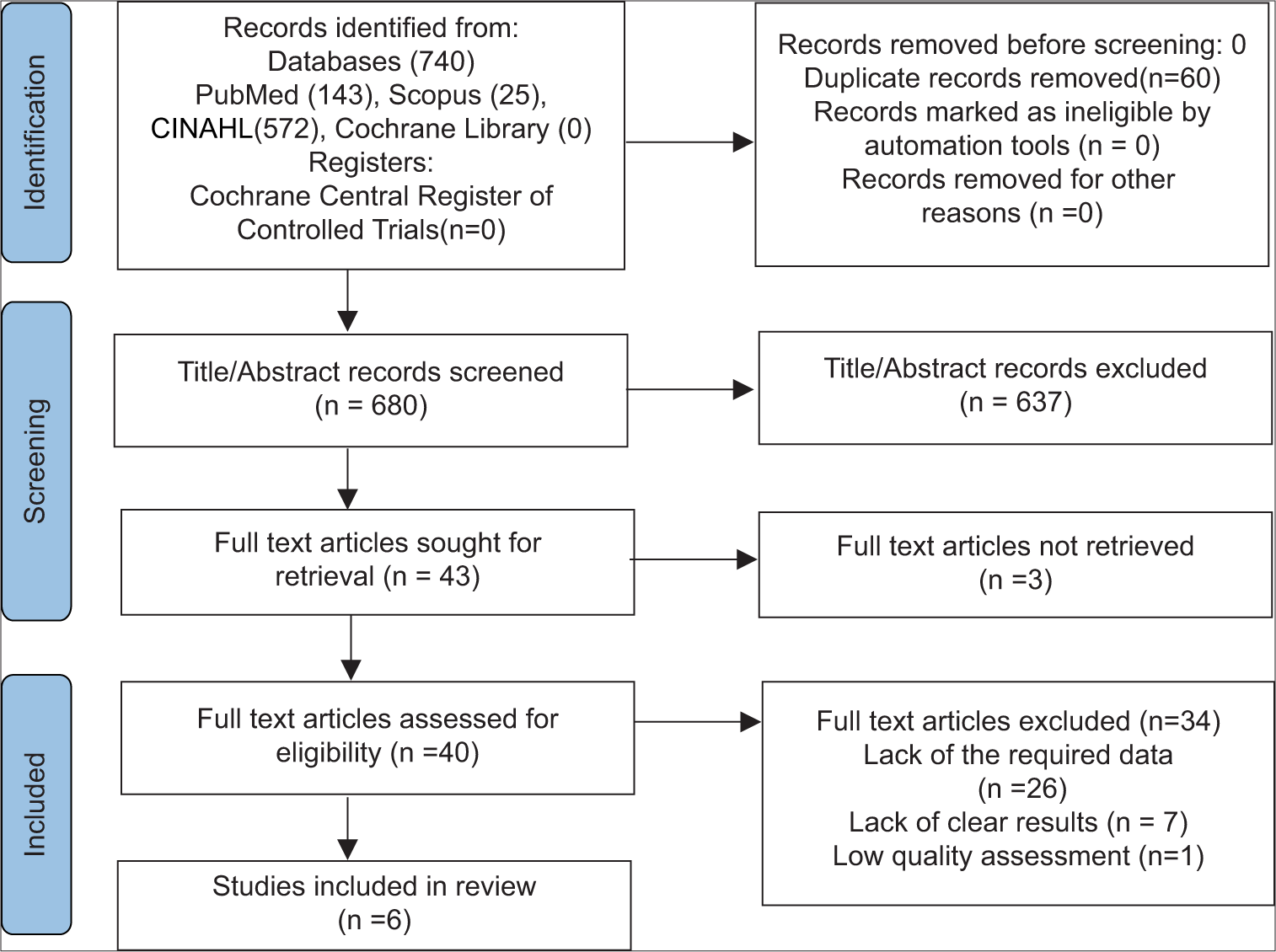
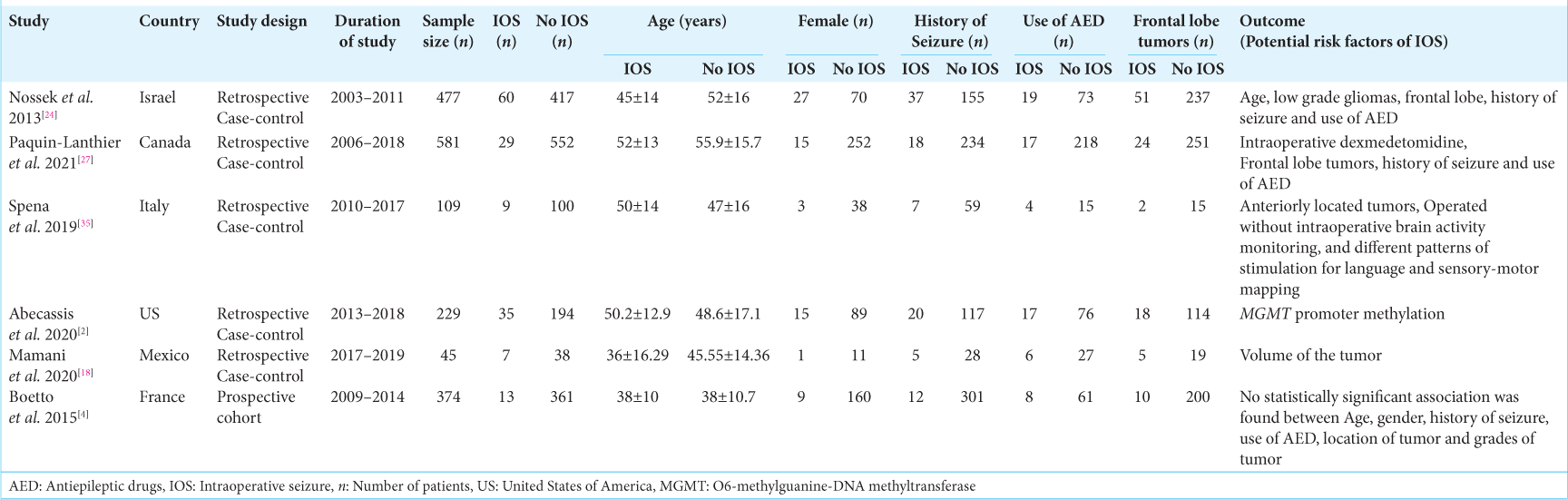
Our initial search strategy yielded 83 publications. Ten publications were read in full text after duplicates were removed and titles and abstracts were checked. Our meta-analysis ultimately comprised a total of six studies with 1815 participants. The thorough screening, eligibility evaluation, and inclusion procedure are displayed [
Figure 1:
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 diagram,[
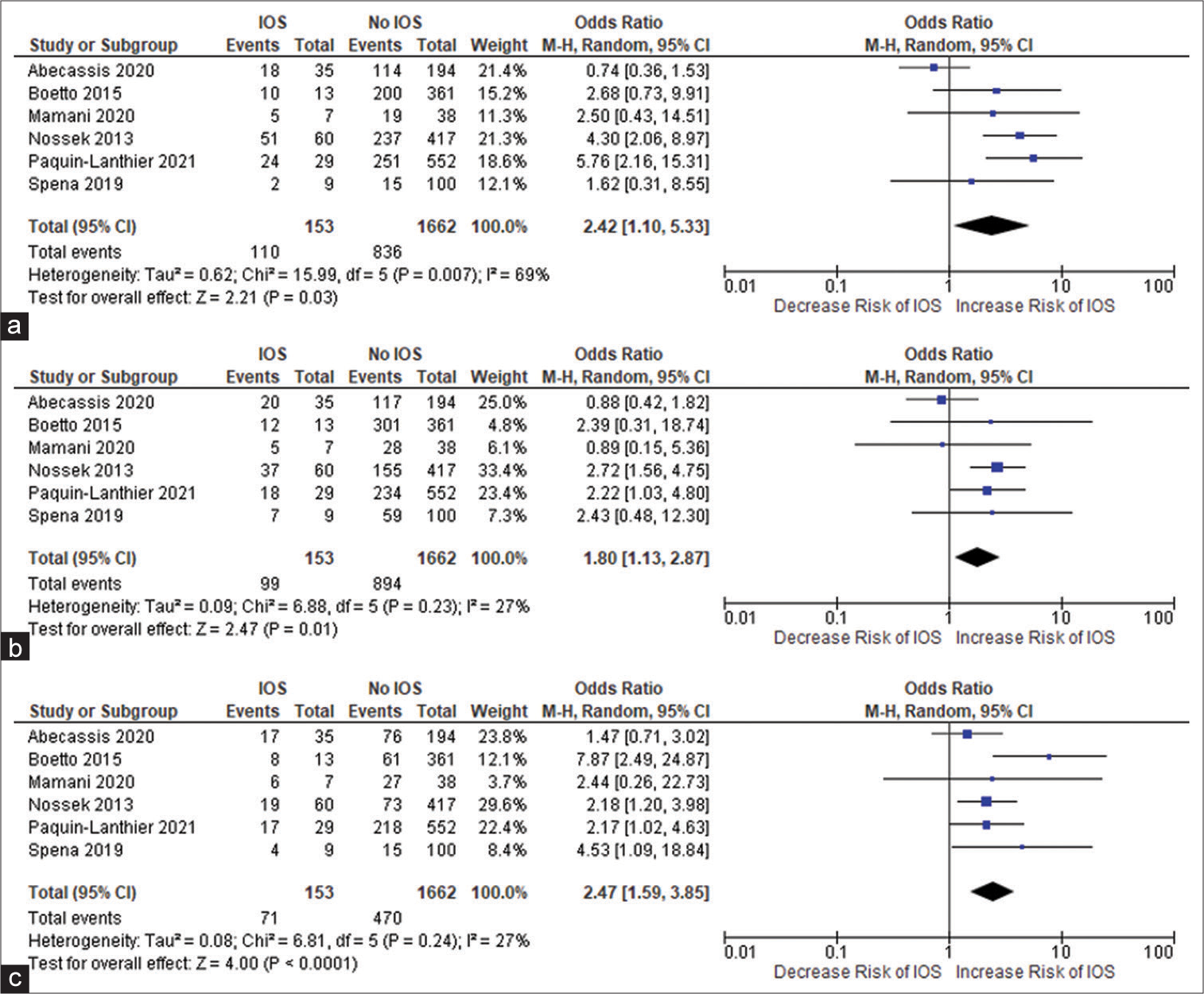
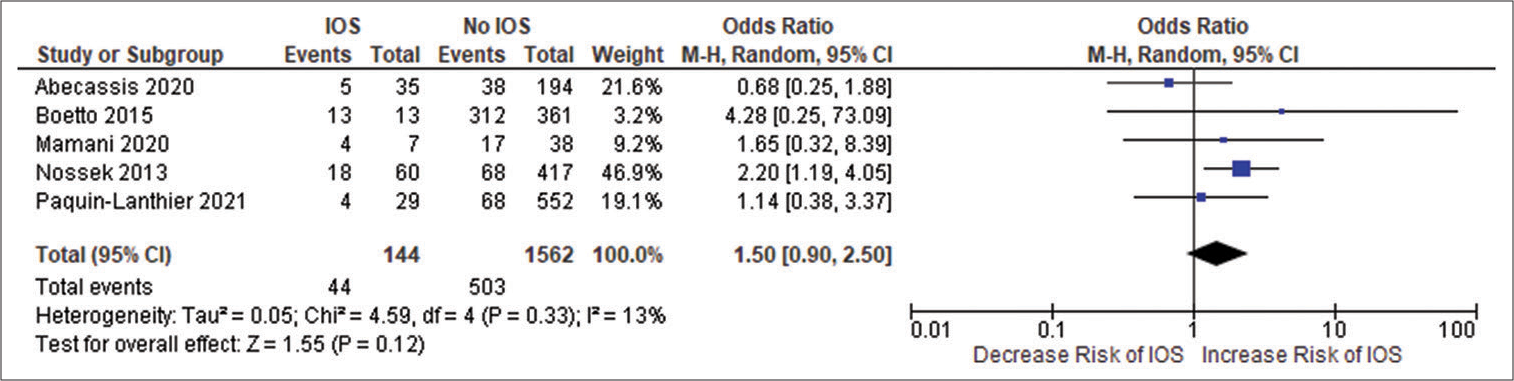
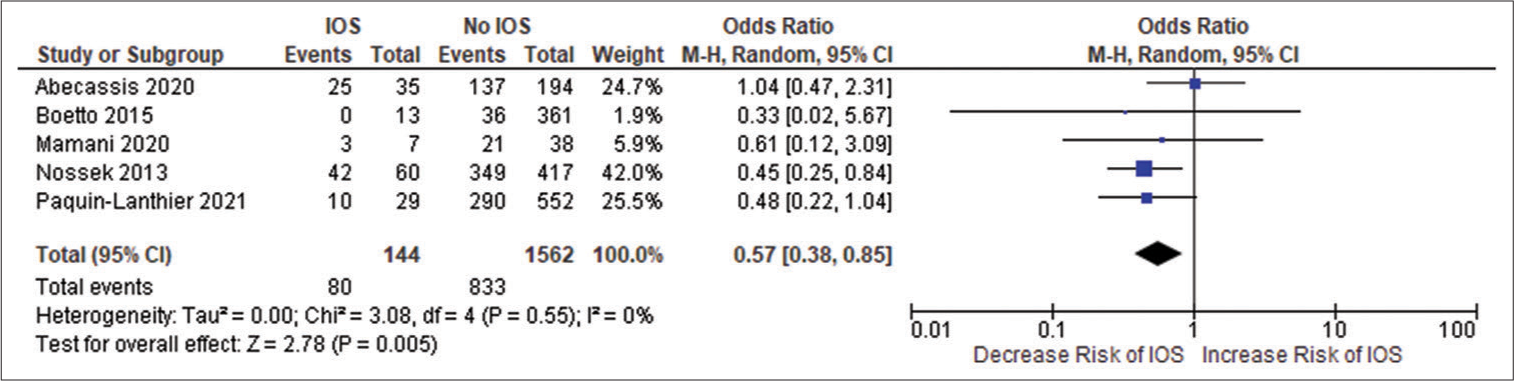
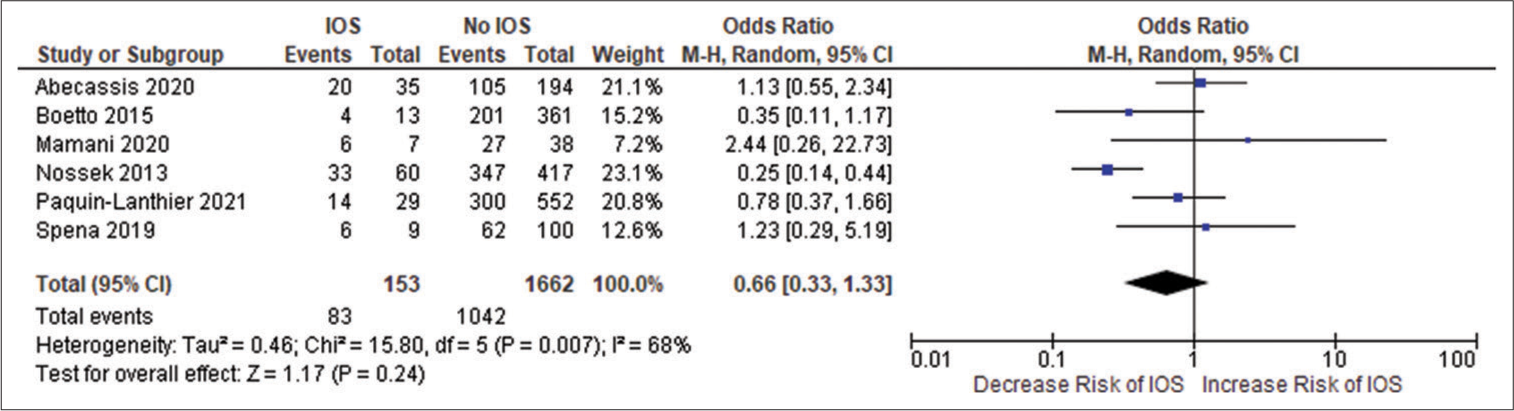
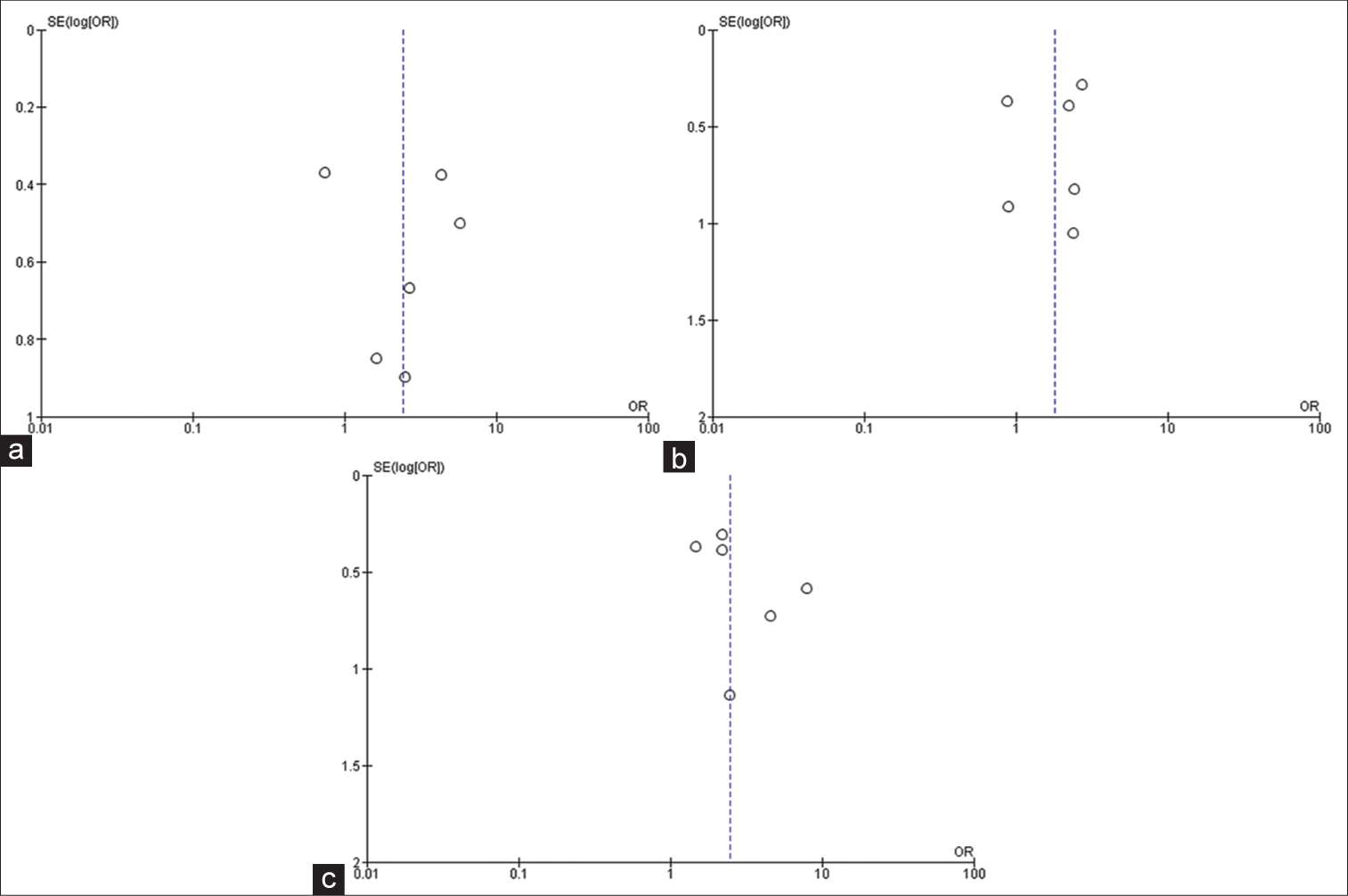
All the included studies reported IOS in patients with a frontal lobe tumor, a history of seizure, and the use of AED, and these variables were statistically significant. A pooled random effect OR of frontal lobe tumors was 2.42 [95% CI: 1.10–5.33, P = 0.03;
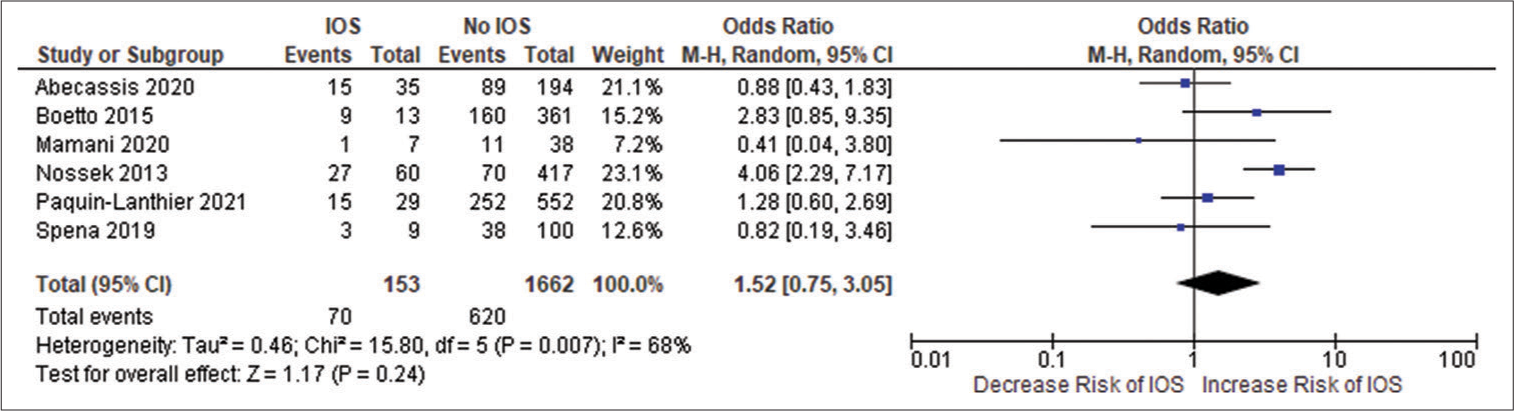
Figure 2:
(a) Forest plot of pooled odds ratio (OR) of frontal lobe tumors. (b) Forest plot of pooled OR of history of seizure. (c) Forest plot of pooled OR of use of antiepileptic drug. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
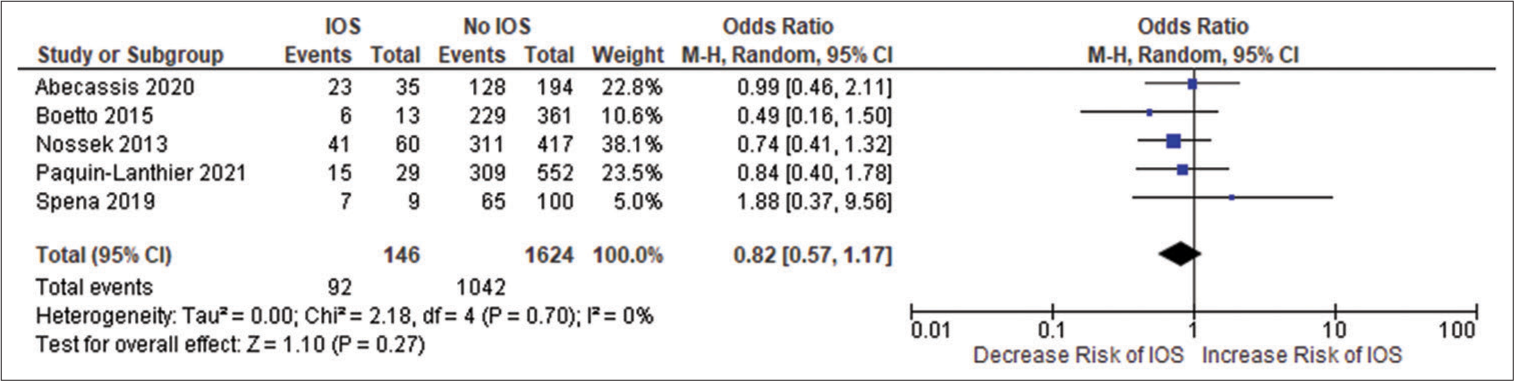
Patients using AEDs had a significant random effect pooled OR of 2.47 [95% CI: 1.59–3.85, P < 0.001;
Figure 2d:
Forest plot of pooled odds ratio of low-grade gliomas. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
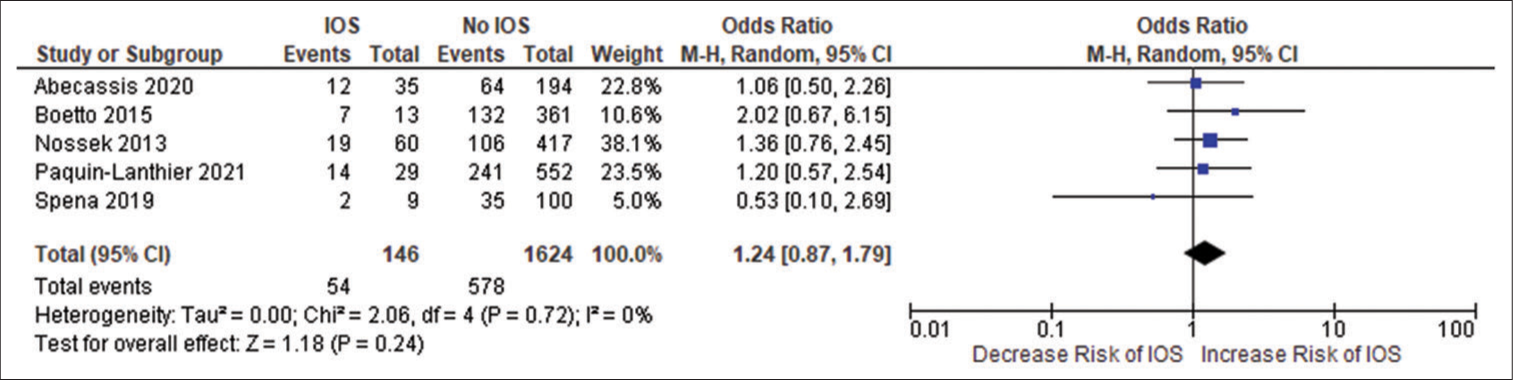
Figure 2e:
Forest plot of pooled odds ratio of high-grade gliomas. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2f:
Forest plot of pooled odds ratio of left hemisphere gliomas. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2g:
Forest plot of pooled odds ratio of right hemisphere gliomas. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2h:
Forest plot of pooled odds ratio of male gender. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2i:
Forest plot of pooled odds ratio of female gender. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2j:
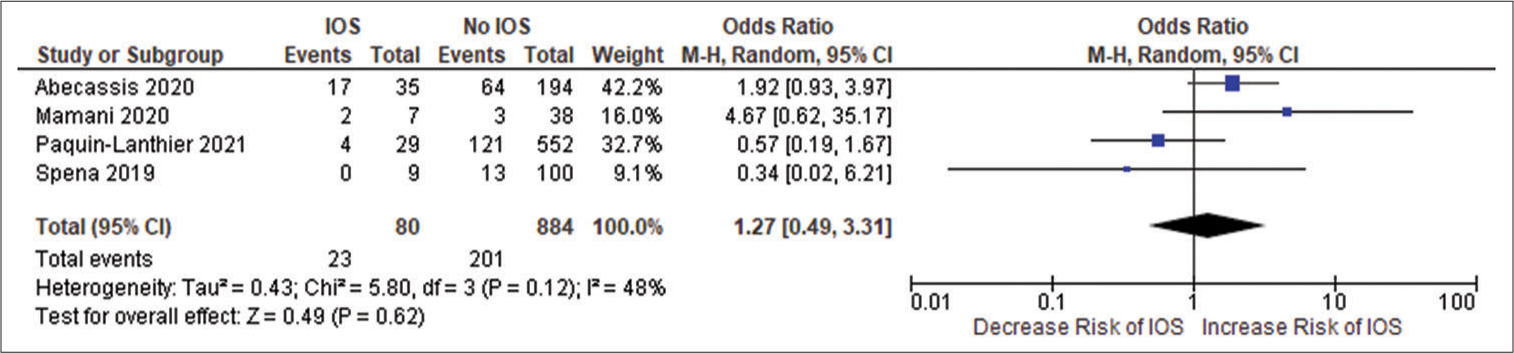
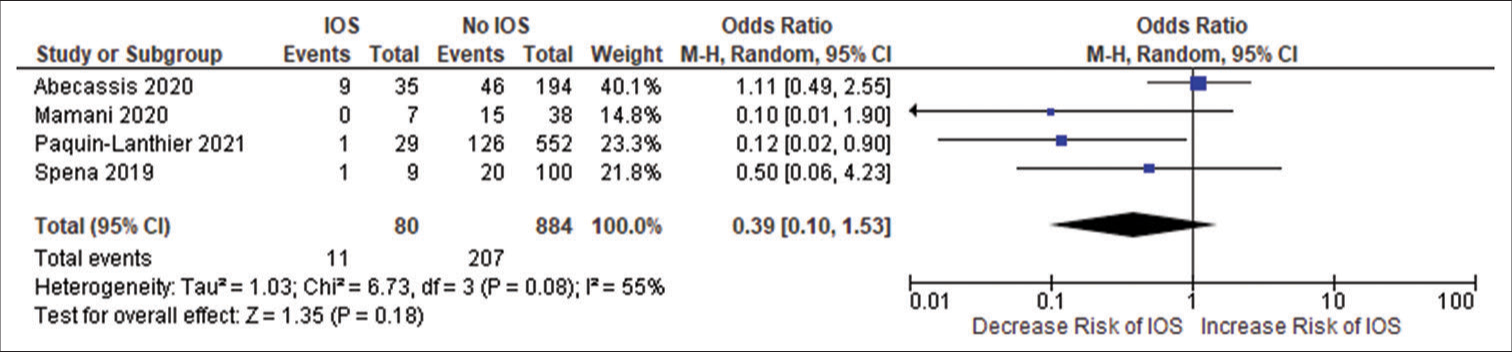
Forest plot of pooled odds ratio of parietal lobe tumor. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2k:
Forest plot of pooled odds ratio of temporal lobe tumor. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each blue quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2l:
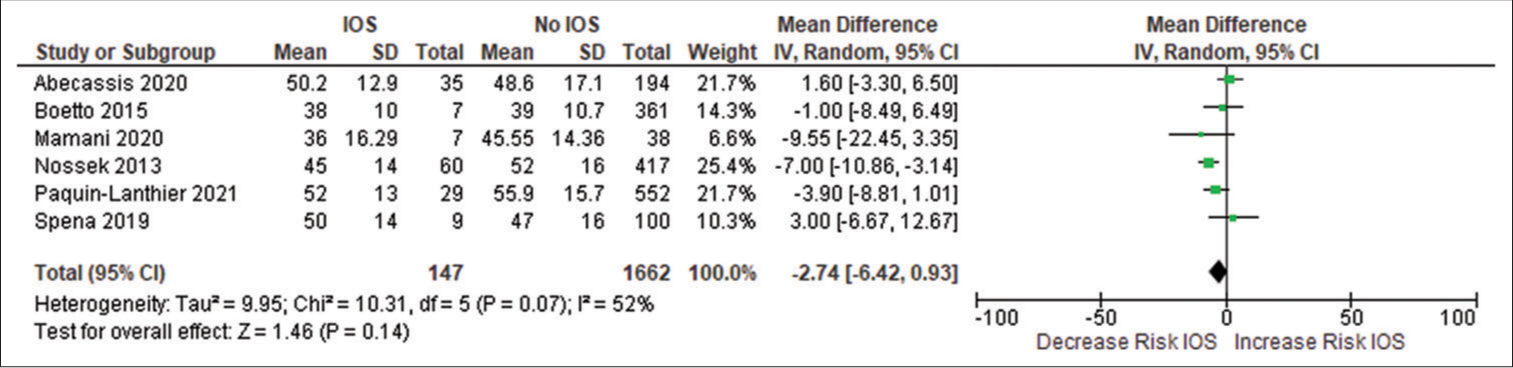
Forest plot of pooled odds ratio of age of patient. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each green quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
Figure 2m:
Forest plot of pooled odds ratio of volume of tumor. IOS: Intraoperative Seizure(s), M-H: Mantel-Haenszel method to estimate pooled odds ratio, CI: Confidence Interval. Each green quadrilateral and adjacent black line represent the study weight and Confidence interval respectively. The black diamond shape box represents the overall effect size.
DISCUSSION
IOSs remain one of the commonly reported complications associated with AC.[
To date, there has been no consensus regarding factors that predict IOS.[
Current literature points toward the importance of lesions in developing IOS, and it is generally accepted that certain brain areas are more predisposed to developing IOS than others, where a frontal lesion is commonly associated with an elevated risk of IOS.[
Tumors have distinct intrinsic epileptogenicity, LGG being the most epileptogenic tumors.[
Patients with a history of seizures preoperatively are at a higher risk of presenting seizures intraoperatively.[
We looked at individuals on AED and we found a strong association between patients on AED and IOS. Although we did not evaluate the number and type of AED the patient was taking and its association with IOS. Age and sex differences in IOS have been debatable in the literature. Although, there is a slight predilection of IOS toward younger age and female gender.[
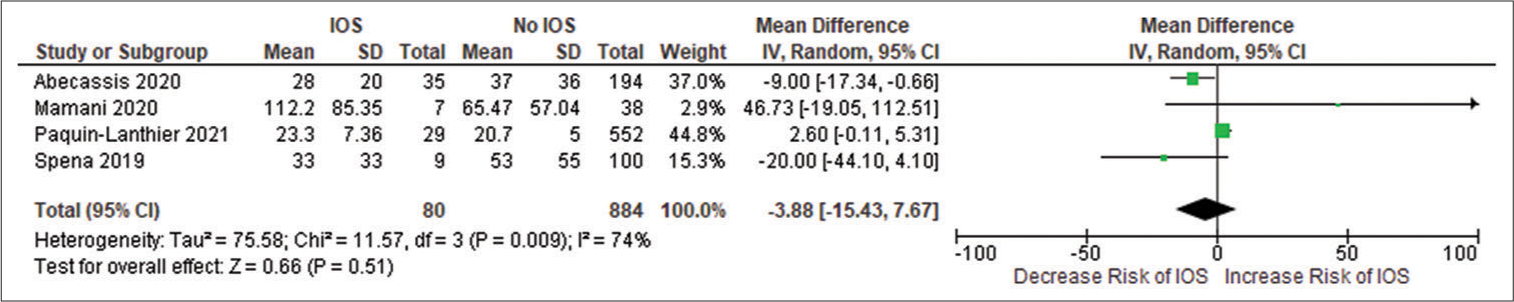
The mean preoperative tumor volume in Eseonu et al.[
Studies have suggested a disparity in the incidence of IOS in AC procedures using different forms of anesthesia.[
Only one study in our analysis studied operative time as a potential risk factor for IOSs[
Limitations
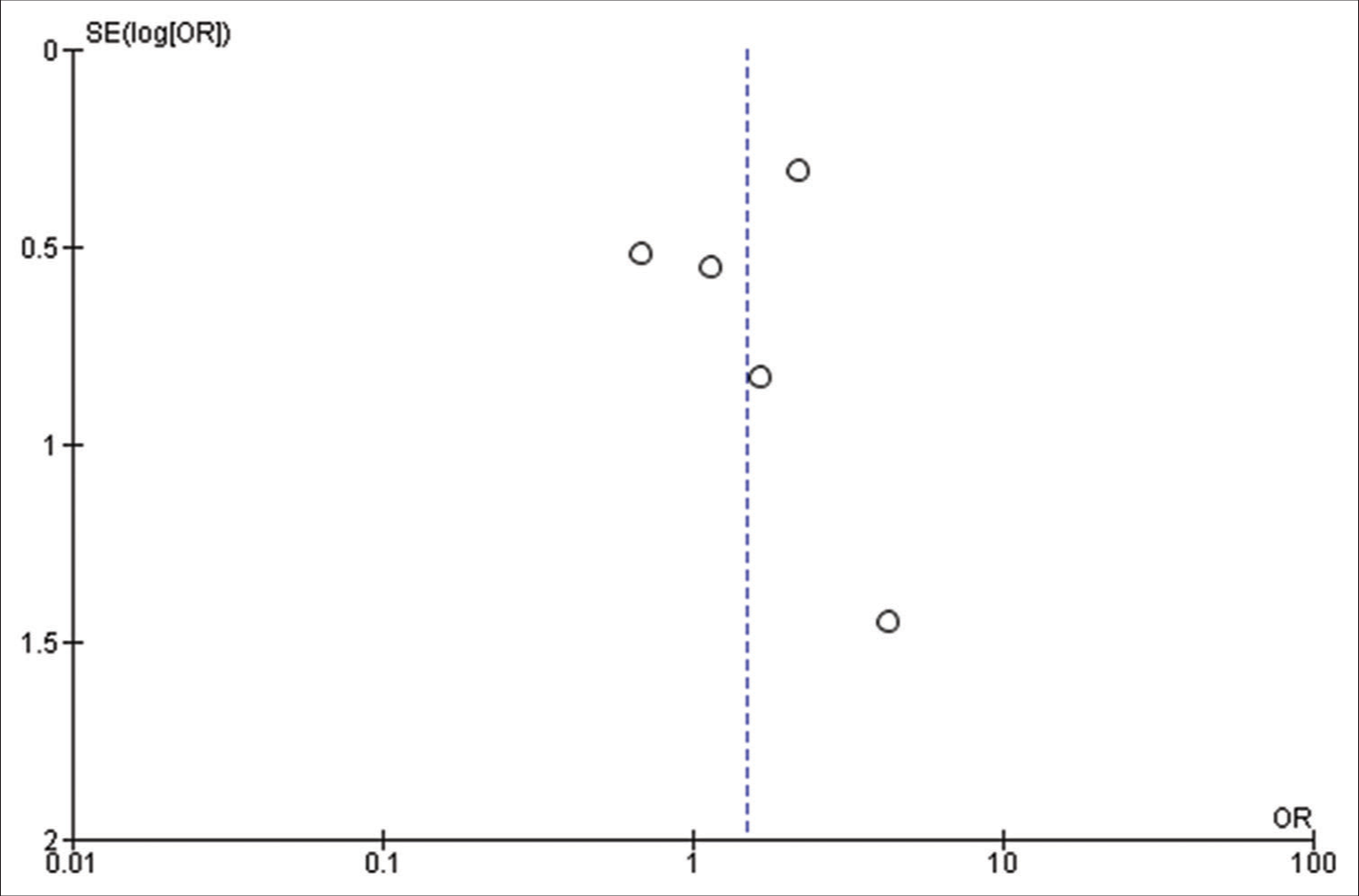
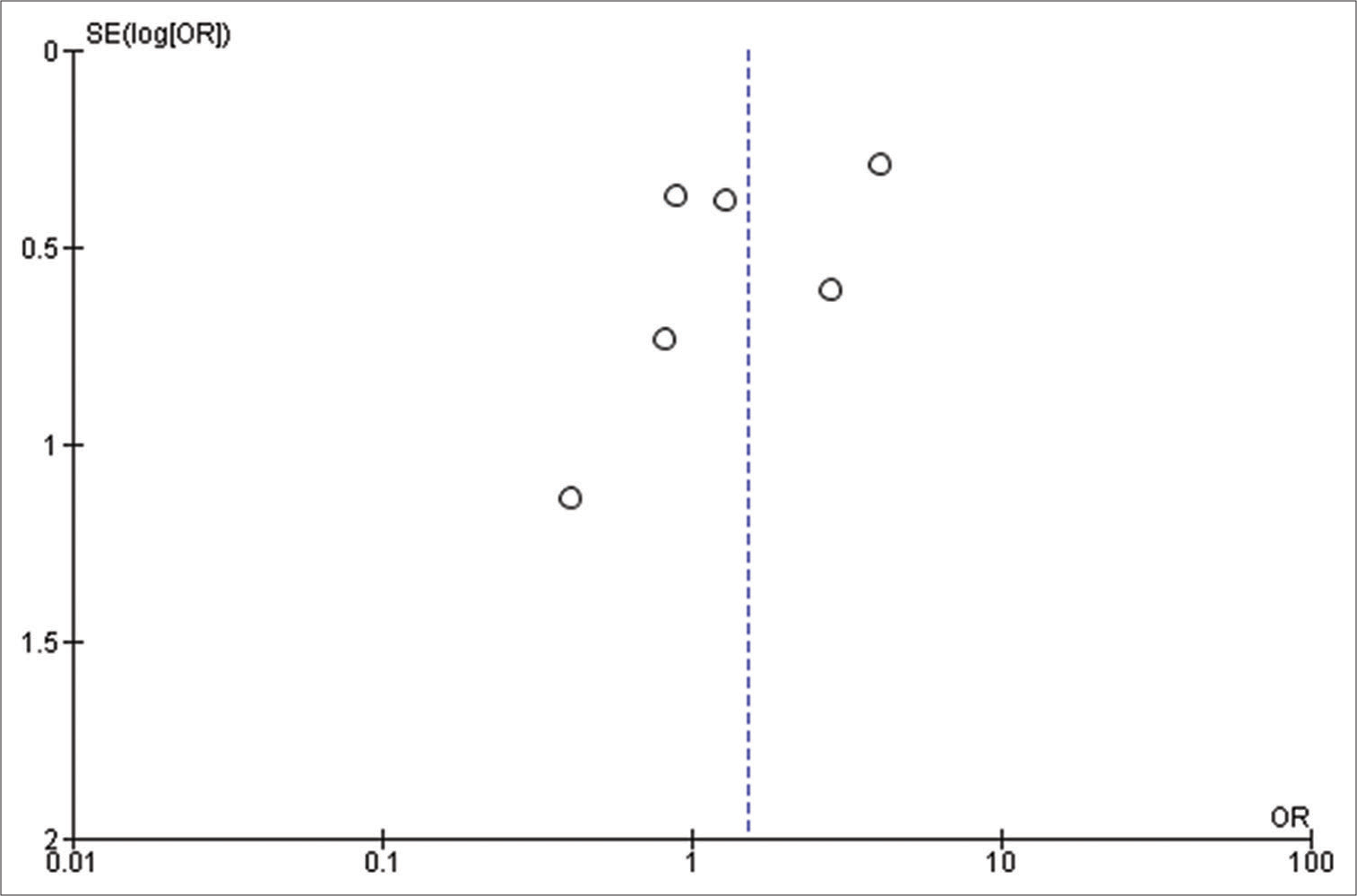
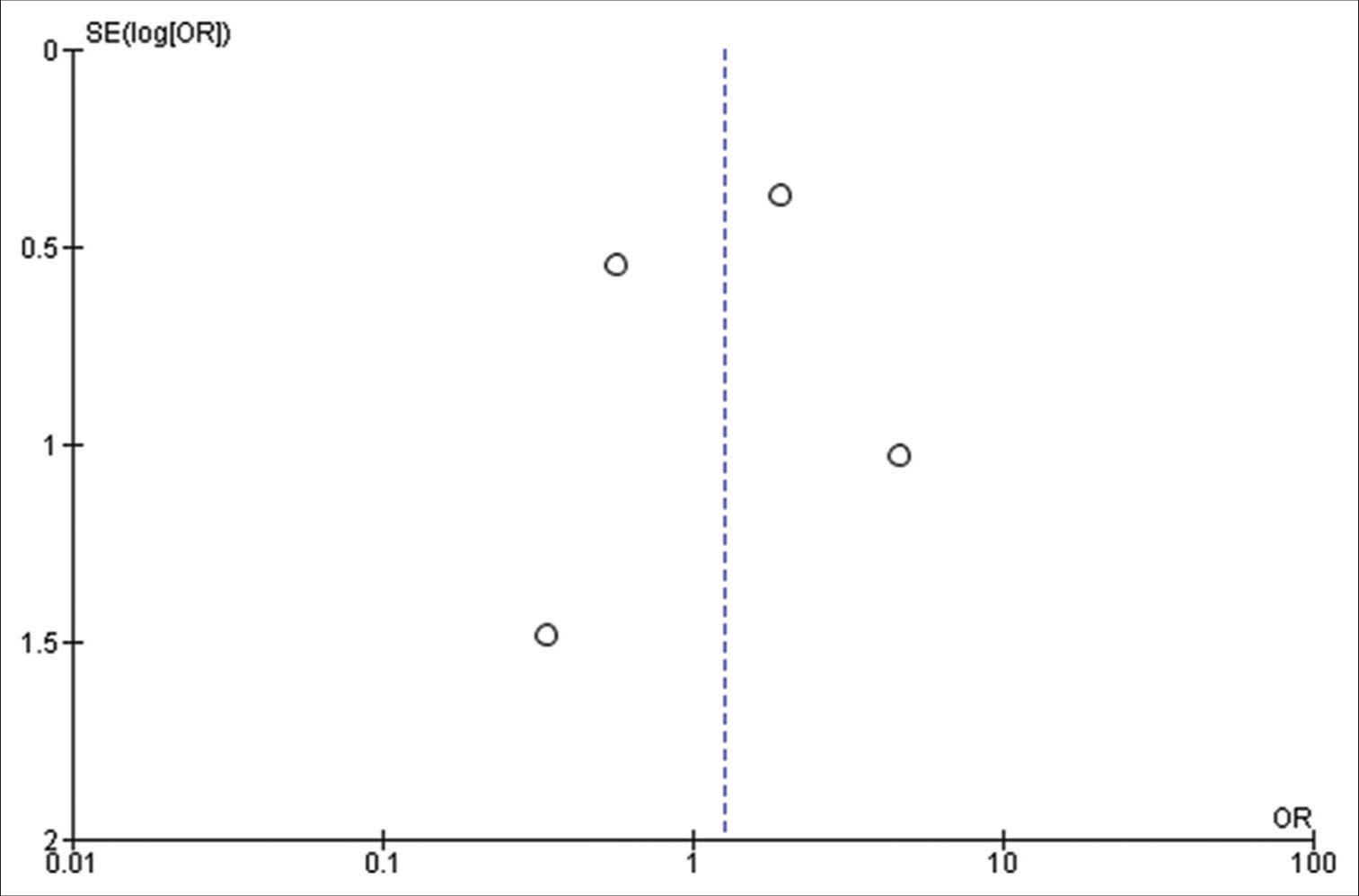
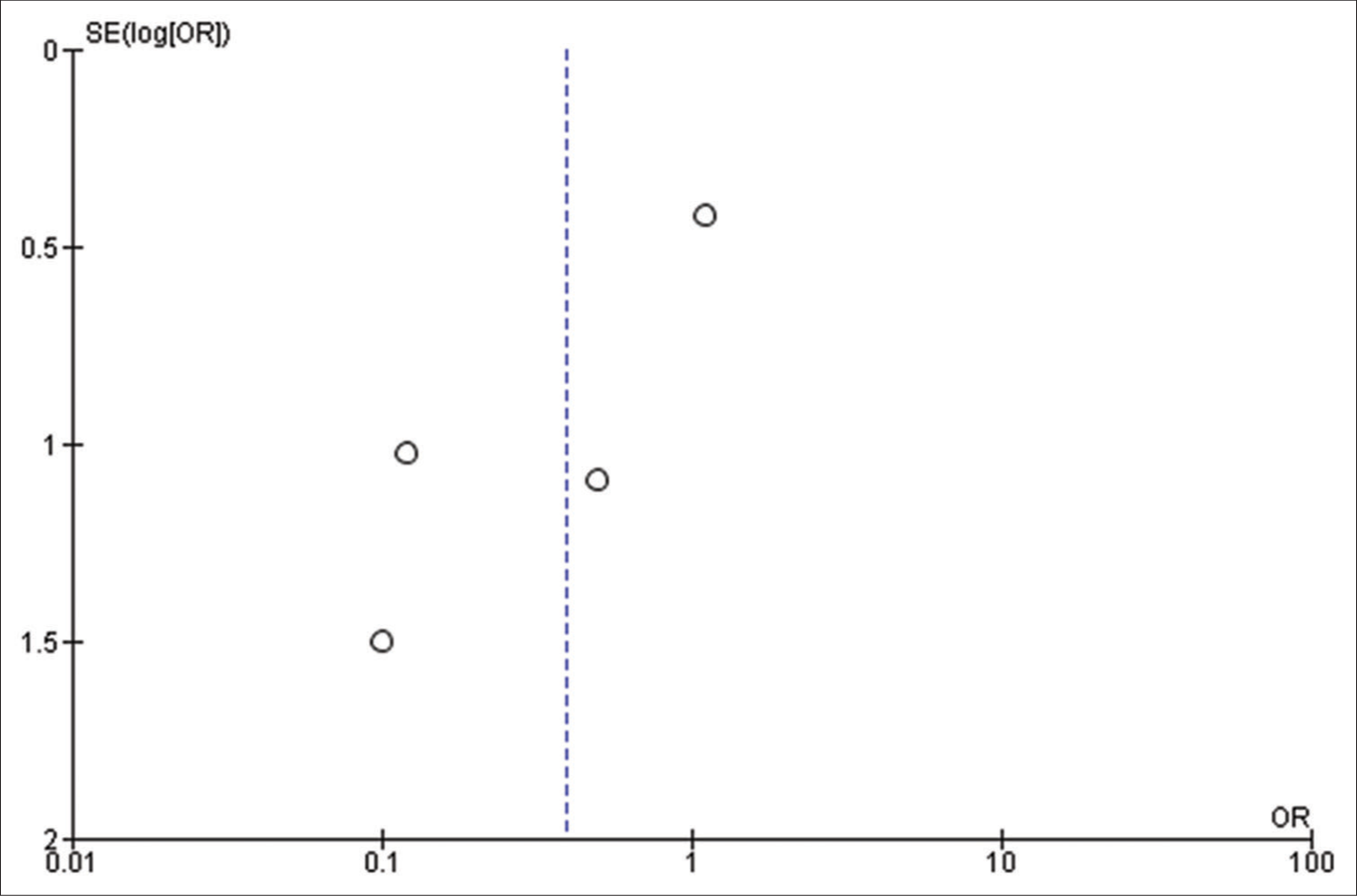
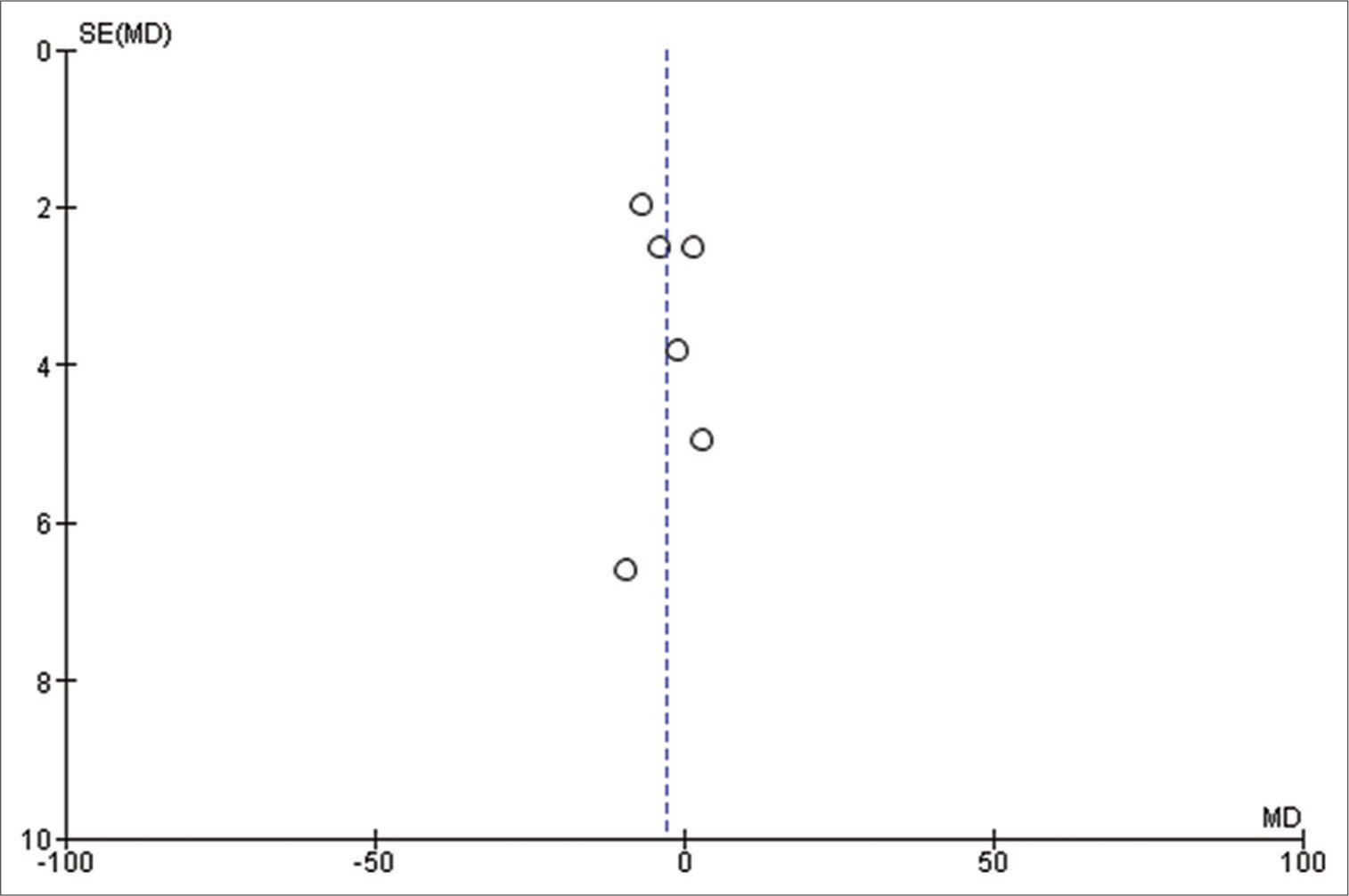
The findings of our meta-analysis are limited by the retrospective nature of included studies and their heterogeneity. Further, analyses such as meta-regression to explore the potential confounders could not be performed appropriately, as we were hindered by the scarcity of relevant studies. In addition, small sample sizes in a few included studies may carry intrinsic publication bias and make the file-drawer issue worse.
We only searched for articles in the English language; therefore, not all the research that met our inclusion criteria may have been found. Studies that implemented intraoperative magnetic resonance imaging guidance for the procedure were also disregarded. The grounds for elimination were due to the facilities’ low generalizability to the hospital, which does not have a complicated infrastructure.
Some of the potential risk factors of IOS, such as preoperative Karnofsky Performance Scale scoring, specific anesthesia regimens for conscious sedation, length of surgery, intensity of current and duration of stimulation during mapping, isocitrate dehydrogenase mutation gliomas, O (6)-methylguanineDNA methyltransferase methylation, and intraoperative use of electrocorticography (ECoG), could not be included in our meta-analysis because of the limited data available.
Moreover, it must be noted that there may have been variations in the studies’ identification of IOSs and other risk variables. Hence, the pooling of these studies was rational, credible, and justifiable despite their discrepancies.
Future direction
Going forward, preliminary steps will comprise small prospective studies to ascertain predictors of IOS. Subsequently, to fully account for the confounders and bias present in retrospective research, a sizable prospective and cohort study and RCT are required. Our meta-analysis results emphasize the need for additional robust data from large prospective cohorts and RCT studies and help fill up the current gaps in the literature available.
CONCLUSION
This study highlights the importance of preoperative recognition of individuals who are more susceptible to IOSs to avoid this devastating AC complication. Patients with lesions of the frontal lobe, a prior seizure history, and patients on AEDs are at higher risk of experiencing IOSs. These factors must be considered during the patient’s preparation for AC. However, it is sometimes difficult to predict IOS precisely, so the presence of a multidisciplinary team, consisting of neurosurgeons, neuroanesthesiologists, Electroencephalography, and ECoG specialists, should be present, prepared to timely detect and intervene to avoid an intractable seizure and consequently a failed AC.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Abaziou T, Tincres F, Mrozek S, Brauge D, Marhar F, Delamarre L. Incidence and predicting factors of perioperative complications during monitored anesthesia care for awake craniotomy. J Clin Anesth. 2020. 64: 109811
2. Abecassis ZA, Ayer AB, Templer JW, Yerneni K, Murthy NK, Tate MC. Analysis of risk factors and clinical sequelae of direct electrical cortical stimulation-induced seizures and after discharges in patients undergoing awake mapping. J Neurosurg. 2020. 134: 1610-7
3. Bartholow R. Experiments on the functions of the human brain. Br Med J. 1874. 1: 727
4. Boetto J, Bertram L, Moulinié G, Herbet G, Moritz-Gasser S, Duffau H. Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: Electrocorticography is not mandatory. World Neurosurg. 2015. 84: 1838-44
5. Brown T, Shah AH, Bregy A, Shah NH, Thambuswamy M, Barbarite E. Awake craniotomy for brain tumor resection: The rule rather than the exception?. J Neurosurg Anesthesiol. 2013. 25: 240-7
6. Brydges G, Atkinson R, Perry MJ, Hurst D, Laqua T, Wiemers J. Awake craniotomy: A practice overview. AANA J. 2012. 80: 61-8
7. Cohen J. A Coefficient of agreement for nominal scales. Educ Psychol Meas. 1960. 20: 37-46
8. De Groot M, Reijneveld JC, Aronica E, Heimans JJ. Epilepsy in patients with a brain tumour: Focal epilepsy requires focused treatment. Brain. 2012. 135: 1002-16
9. Eseonu CI, Rincon-Torroella J, Lee YM, Refaey K, Tripathi P, Quinones-Hinojosa A. Intraoperative seizures in awake craniotomy for perirolandic glioma resections that undergo cortical mapping. J Neurol Surg A Cent Eur Neurosurg. 2018. 79: 239-46
10. Gonen T, Grossman R, Sitt R, Nossek E, Yanaki R, Cagnano E. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J Neurosurg. 2014. 121: 1133-8
11. Herrick IA, Craen RA, Gelb AW, McLachlan RS, Girvin JP, Parrent AG. Propofol sedation during awake craniotomy for seizures: Electrocorticographic and epileptogenic effects. Anesth Analg. 1997. 84: 1280-4
12. Hervey-Jumper SL, Li J, Lau D, Molinaro AM, Perry DW, Meng L. Awake craniotomy to maximize glioma resection: Methods and technical nuances over a 27-year period. J Neurosurg. 2015. 123: 325-39
13. Ibrahim GM, Bernstein M. Awake craniotomy for supratentorial gliomas: Why, when and how?. CNS Oncol. 2012. 1: 71-83
14. Jones H, Smith M. Awake craniotomy. Contin Educ Anaesth Crit Care Pain. 2004. 4: 189-92
15. July J, Manninen P, Lai J, Yao Z, Bernstein M. The history of awake craniotomy for brain tumor and its spread into Asia. Surg Neurol. 2009. 71: 621-4
16. Kim SS, McCutcheon IE, Suki D, Weinberg JS, Sawaya R, Lang FF. Awake craniotomy for brain tumors near eloquent cortex: Correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery. 2009. 64: 836-45
17. Kwinta BM, Myszka AM, Bigaj MM, Krzyżewski RM, Starowicz-Filip A. Intra-and postoperative adverse events in awake craniotomy for intrinsic supratentorial brain tumors. Neurol Sci. 2021. 42: 1437-41
18. Mamani R, Jacobo JA, Mejia S, Nuñez-Velasco S, AragonArreola J, Moreno S. Analysis of intraoperative seizures during bipolar brain mapping in eloquent areas: Intraoperative seizures in brain mapping. Clin Neurol Neurosurg. 2020. 199: 106304
19. Manninen PH, Tan TK. Postoperative nausea and vomiting after craniotomy for tumor surgery: A comparison between awake craniotomy and general anesthesia. J Clin Anesth. 2002. 14: 279-83
20. McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA. 2018. 319: 388-96
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009. 151: 264-9
22. Natalini D, Ganau M, Rosenkranz R, Petrinic T, Fitzgibbon K, Antonelli M. Comparison of the asleep-awake-asleep technique and monitored anesthesia care during awake craniotomy: A systematic review and meta-analysis. J Neurosurg Anesthesiol. 2022. 34: e1-13
23. Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T. Failed awake craniotomy: A retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J Neurosurg. 2013. 118: 243-9
24. Nossek E, Matot I, Shahar T, Barzilai O, Rapoport Y, Gonen T. Intraoperative seizures during awake craniotomy: Incidence and consequences: Analysis of 477 patients. Neurosurgery. 2013. 73: 135-40
25. Pace A, Bove L, Innocenti P, Pietrangeli A, Carapella CM, Oppido P. Epilepsy and gliomas: Incidence and treatment in 119 patients. J Exp Clin Cancer Res. 1998. 17: 479-82
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 37: n71
27. Paquin-Lanthier G, Subramaniam S, Leong KW, Daniels A, Singh K, Takami H. Risk factors and characteristics of intraoperative seizures during awake craniotomy: A retrospective cohort study of 562 consecutive patients with a space-occupying brain lesion. J Neurosurg Anesthesiol. 2023. 35: 194-200
28. Pedder H, Sarri G, Keeney E, Nunes V, Dias S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst Rev. 2016. 5: 212
29. Potters JW, Klimek M. Awake craniotomy: Improving the patient’s experience. Curr Opin Anaesthesiol. 2015. 28: 511-6
30. Roca E, Pallud J, Guerrini F, Panciani PP, Fontanella M, Spena G. Stimulation-related intraoperative seizures during awake surgery: A review of available evidences. Neurosurg Rev. 2020. 43: 87-93
31. Sacko O, Lauwers-Cances V, Brauge D, Sesay M, Brenner A, Roux FE. Awake craniotomy vs surgery under general anesthesia for resection of supratentorial lesions. Neurosurgery. 2011. 68: 1192-8
32. Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008. 358: 18-27
33. Singh K, Dua A, editors. Anesthesia for awake craniotomy. StatPearls. Treasure Island, FL: StatPearls; 2022. p.
34. Sokhal N, Rath GP, Chaturvedi A, Dash HH, Bithal PK, Chandra PS. Anaesthesia for awake craniotomy: A retrospective study of 54 cases. Indian J Anaesth. 2015. 59: 300-5
35. Spena G, Roca E, Guerrini F, Panciani PP, Stanzani L, Salmaggi A. Risk factors for intraoperative stimulation-related seizures during awake surgery: An analysis of 109 consecutive patients. J Neurooncol. 2019. 145: 295-300
36. Spena G, Schucht P, Seidel K, Rutten GJ, Freyschlag CF, D’Agata F. Brain tumors in eloquent areas: A european multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg Rev. 2017. 40: 287-98
37. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010. 25: 603-5
38. Stevanovic A, Rossaint R, Veldeman M, Bilotta F, Coburn M. Anaesthesia management for awake craniotomy: Systematic review and meta-analysis. PLoS One. 2016. 11: e0156448
39. Van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: Epidemiology, mechanisms, and management. Lancet Neurol. 2007. 6: 421-30
40. Wang YC, Lee CC, Takami H, Shen S, Chen KT, Wei KC. Awake craniotomies for epileptic gliomas: Intraoperative and postoperative seizure control and prognostic factors. J Neurooncol. 2019. 142: 577-86
41. Zhang K, Gelb AW. Awake craniotomy: Indications, benefits, and techniques. Colombian J Anesthesiol. 2018. 46: 46-51