- Department of Neurosurgery, Kanazawa Medical Center, Shimoishibikimachi, Kanazawa, Ishikawa, Japan.
DOI:10.25259/SNI_2_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Yu Shimizu, Katsuhiro Tsuchiya, Norihiro Fujisawa. Risk factors of diffuse alveolar hemorrhage after acute ischemic stroke treated with tissue-type plasminogen activator. The effectiveness of activated recombinant factor VII treatment. 30-May-2020;11:129
How to cite this URL: Yu Shimizu, Katsuhiro Tsuchiya, Norihiro Fujisawa. Risk factors of diffuse alveolar hemorrhage after acute ischemic stroke treated with tissue-type plasminogen activator. The effectiveness of activated recombinant factor VII treatment. 30-May-2020;11:129. Available from: https://surgicalneurologyint.com/surgicalint-articles/10056/
Abstract
Background: Diffuse alveolar hemorrhage (DAH) is a rare and frequently life-threatening complication of a variety of conditions. DAH may result from coagulation disorders, inhaled toxins, or infections. We report a series of patients who developed DAH after receiving a tissue-type plasminogen activator (tPA) for acute cerebral infarction. We aimed to find risk factors of DAH in patients receiving tPA and the effectiveness of activated recombinant factor VII (rFVIIa) treatment for the same.
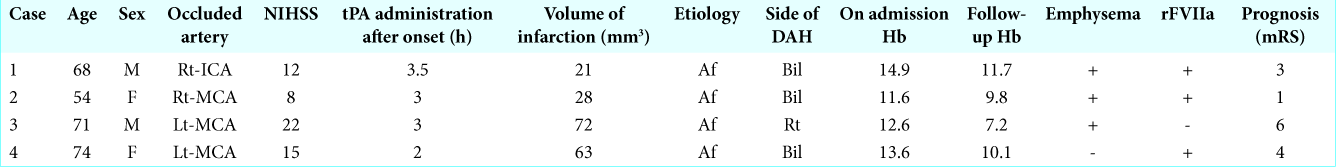
Case Description: A total of 1023 acute ischemic stroke (AIS) patients who received tPA in our department from January 2006 to December 2018 were enrolled in this study. Four of the 1023 patients (0.39%) developed DAH. The modified Rankin scale was used to assess clinical severity. Infarction volume was assessed upon follow-up using DWI (diffusion-weighted imaging). Atherothrombotic brain infarction cases were excluded from the study. The age, sex, occlusion site, area of infarction, emphysema, intracranial hemorrhage, and neurological outcomes were analyzed. Patients who developed DAH were more likely to have a history of emphysema. We administered rFVIIa to three DAH patients with good prognosis.
Conclusion: The inclusion/exclusion criteria of tPA were based on the AHA/ASA Guidelines for the early management of patients with AIS.These patients had no evidence of infections, bronchoscopy, autoimmune diseases, HIV, and transplantations. Our study suggests that systemic administration of rFVIIa for DAH is effective. Emphysema may be a risk factor for the development of DAH following tPA. When we use tPA for emphysema patients, we must be careful about DAH enough.
Keywords: Activated recombinant factor VII, Acute ischemic stroke, Diffuse alveolar hemorrhage, National Institutes of Health Stroke Scale, Tissue-type plasminogen activator
INTRODUCTION
Diffuse alveolar hemorrhage is an uncommon but acute and life-threatening event. A number of diseases can cause pulmonary bleeding, and it can accompany Wegener granulomatosis, microscopic polyangiitis, Goodpasture syndrome, connective tissue disorders, antiphospholipid antibody syndrome, infectious or toxic exposures, and neoplastic conditions.[
CASE DESCRIPTION
Case 1
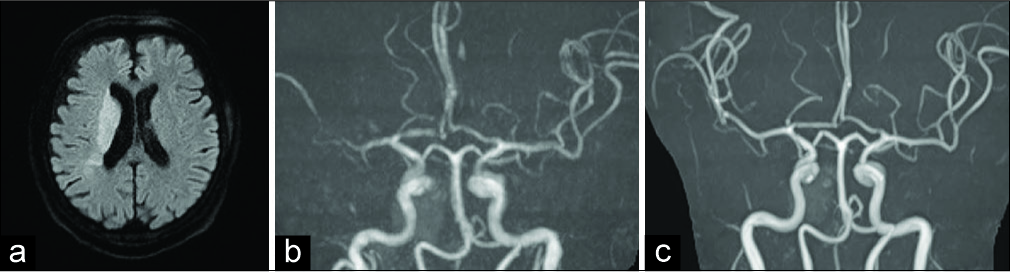
A 68-year-old man with the left hemiparesis from 2 h previously visited the emergency room. His medical history included hypertension and bilateral emphysema due to heavy smoking. Vital sign assessment revealed tachycardia; examination of the heart revealed atrial fibrillation (AF). Neurological examination revealed left hemiparesis and mild disturbance of consciousness. The National Institutes of Health Stroke Scale (NIHSS) score was 12. A magnetic resonance imaging (MRI) (diffusion-weighted image) showed right corona radiate infarction [
Case 2
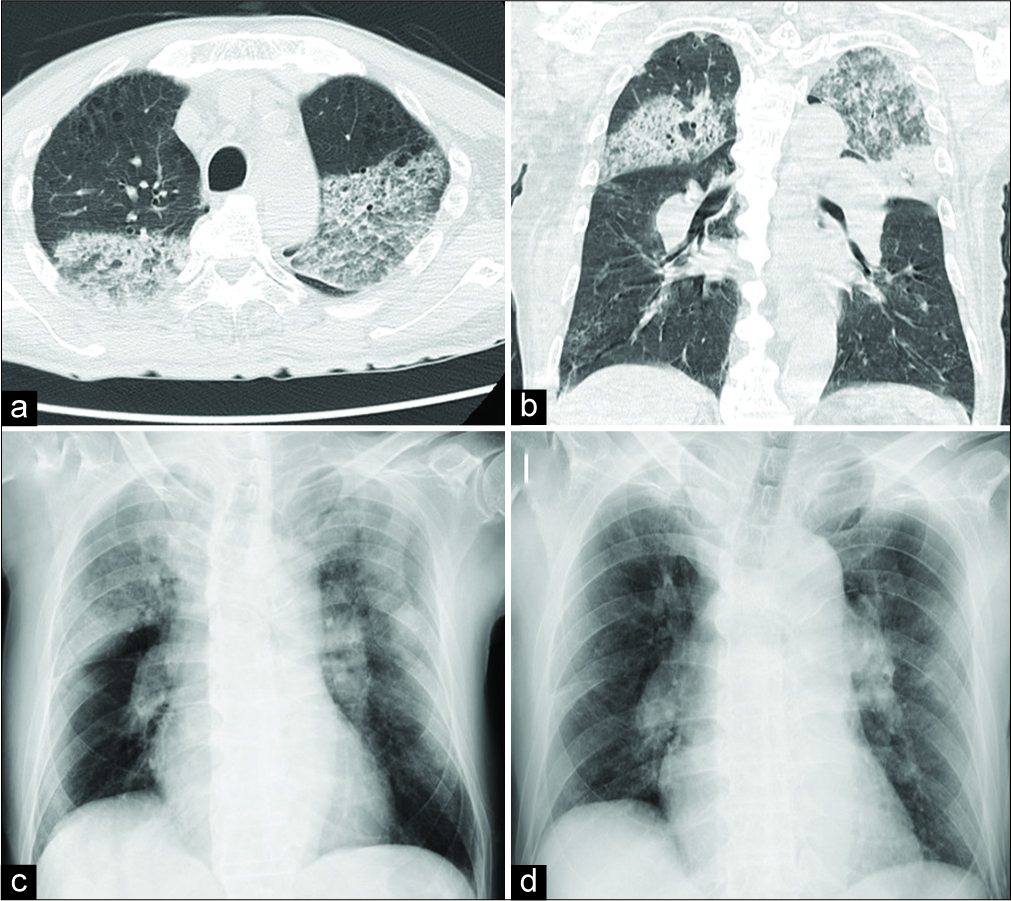
A 54-year-old male, previously healthy, nonsmoking, presented with the right hemiparesis from 1.5 h with a body temperature of 36.1°C and room air oxygen saturation of 95%. Cardiac examination showed arterial fibrillation. His white blood cell count was 6.3 × 109 cells/L, hematocrit was 39%, and platelet count was 196 × 109 cells/L. The APTT was not prolonged at 35 s and PT and INR were normal. Serologies for ANA, c- and p-ANCA, and anti- GBM antibodies were negative. IgG ACA and anti-2GPI antibodies were persistently negative. Urinalysis was normal. The NIHSS score was 10. Initial brain CT excluded bleeding. tPA was administered 3 h after symptom onset. After tPA, there was no improvement in the NIHSS score. Six hours after ITT, he presented with acute dyspnea, hypoxemia, and hemoptysis. Chest radiography showed bilateral alveolar infiltrates and emphysema, but there were no pulmonary emboli seen on CT evaluation of the pulmonary vasculature. His hemoglobin level dropped from 11.6 to 9.8 g/dL. Bronchoscopy revealed DAH. He was diagnosed as having tPA-associated alveolar hemorrhage and treated by administering intrapulmonary rFVIIa (75 mg/kg) with corticosteroids. Within 5 days, he had near- complete resolution of symptoms and radiographic findings. After 3 weeks, he was extubated and discharged with an mRS score of 2 at the end of 1 week. A control thorax CT performed after 6 months showed complete resolution of the infiltrates, and the mRS score was 1.
Case 3
A 71-year-old female was admitted to the emergency room 90 min after acute onset of the right hemiplegia and global aphasia. Her medical history revealed that she had mitral stenosis and AF for several years. She was not using an anticoagulant drug. The NIHSS score was 22. Initial brain CT excluded bleeding. Initial laboratory findings showed no abnormality in routine biochemical, coagulation, and complete blood count parameters. tPA was administered 3 h after symptom onset. After tPA administration, there was no improvement in the NIHSS score. Follow-up brain CT at 24 h showed a large ischemic lesion in the area of the left MCA.
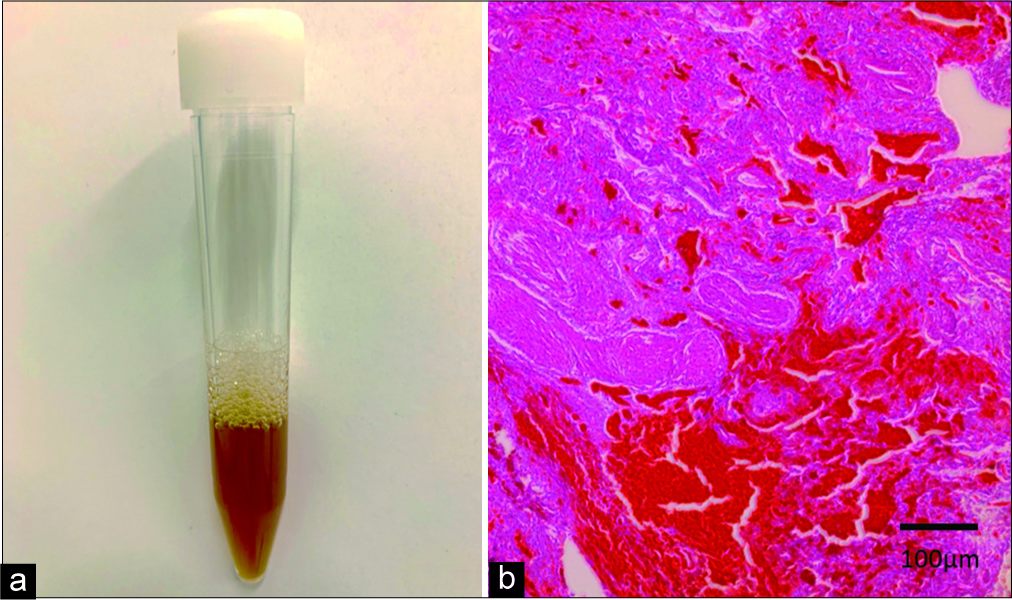
Eight hours after tPA, she was experiencing shortness of breath, an oxygen saturation on room air of 88%, and bilateral rales. Her hemoglobin level dropped from 12.5 to 7.2 g/dL. She received 4 units of fresh frozen plasma and 2 units of packed red blood cells. Chest radiographs showed new bilateral alveolar infiltrates and no evidence of pulmonary emboli. We performed bronchoscopy with bronchial alveolar lavage (BAL). The returned lavage fluid was bloody from aliquot to aliquot [
Figure 3:
(a) Bronchoalveolar lavage (BAL) was performed in the left upper lobe (Case 3). We observed progressive hemorrhagic BAL. BAL fluid analysis shows an increase in red blood cells. (b) Autopsy of the lung tissue (×20) (Case 3) showing inflammatory cell infiltrate of septae and extravasation of red blood cells into alveolar spaces.
Case 4
A 75-year-old male presented with the right hemiparesis and motor aphasia from 1 h. He had not previously been diagnosed with undifferentiated connective tissue disease. He had a 50 pack-year smoking history. His medical history revealed that he had prosthetic mitral valve AF detected on electrocardiogram. Initial laboratory findings showed no abnormality in routine biochemical, coagulation, and complete blood count parameters. The NIHSS score was 15. Initial brain CT had not shown intracranial bleeding. Two hours after symptom onset, tPA was administered. Five hours after tPA administration, there was improvement in the NIHSS score (15–10). He was afebrile and had a room air oxygen saturation of 84%. Basilar rales were present on lung examination. Chest radiography showed bilateral alveolar infiltrates and emphysema; CT scan of the chest showed no pulmonary emboli. Bronchoscopy was significant for bilateral alveolar hemorrhage without evidence of infection. His hemoglobin level dropped from 13.6 to 10.1 g/dL. He received 4 units of packed red blood cells. Control chest X-ray performed after 1 month showed complete resolution of the infiltrates. The patient was transferred to palliative care unit 1 month after tPA with a modified Rankin scale (mRS) score of 4.
DISCUSSION
Thrombolytic therapy induces a marked hemostatic defect by combined actions on blood components, the vessel wall, and the hemostatic plug resulting in hemorrhagic complications in the central nervous system, GI and GU tracts, retroperitoneum, and at vascular access sites.[
Symptomatic intracerebral hemorrhage is a well-known complication of antiplatelet or anticoagulant therapy; however, there are limited data regarding extracranial hemorrhage, especially DAH in AIS. Alveolar hemorrhage (AH) is characterized by accumulation of red blood cells within acinar lung units derived from alveolar capillaries or venules.[
The cause of our patients’ intra-alveolar bleeding remains unknown. However, it was interesting that most of our patients had emphysema. Prior emphysema might have been a risk factor for this adverse effect. Fibrinolysis induced by tPA causes hemostatic plug disintegration and bleeding from sites of emphysema. It is possible, however, that prior emphysema contributed to tPA-related bleeding because of the presence of fragile line blood vessels (angiogenesis) in the scar.
Diffuse alveolar hemorrhage syndrome has three main histological appearances: (1) pulmonary capillaritis characterized by neutrophilic infiltration of the interstitium leading to capillary damage; (2) bland hemorrhage into the alveolar space without inflammation; and (3) diffuse alveolar damage similar to acute respiratory distress syndrome.
In the third pattern, there is hemorrhage in alveolar spaces with alveolar damage. This pattern is associated with anticoagulant therapy, idiopathic pulmonary hypertension, and emphysema.[
The most important laboratory finding is the decrease in hematocrit, especially in acute-onset patients. Anti- neutrophil cytoplasmic antibodies, anti-double-stranded DNA, antinuclear antibodies, anti-GBM antibodies, coagulation studies, and peripheral smear should be assessed for diagnosis and urinalysis; serum urea and creatinine should be assessed for renal involvement.[
Our study demonstrates low mortality rate after administering intrapulmonary rFVIIa [
Data from 10 cases with MI showed that DAH occurred within 3 h–12 h following ITT and that previous pneumonia, pulmonary, or cardiac catheterization, defibrillation or cardiopulmonary resuscitation, arrhythmia, heart failure, and chronic obstructive pulmonary disease such as emphysema were comorbidities that increased the development of DAH.[
Only one patient died within 1 week of massive DAH and respiratory failure. The other three patients survived, and DAH recovered completely. The most important laboratory finding of DAH, decreased hemoglobin, was observed in all patients. We believe that the use of rFVIIa may be effective in the treatment of DAH following ITT in patients with AIS. In this study, rFVIIa administration was very effective. Development of DAH due to tPA in three patients with AIS with emphysema and showing effectiveness of rFVIIa was reported for the 1st time. Systemic administration of FVIIa for use for the uncontrollable life-threatening hemorrhage may become an effective treatment even in patients with AIH.
CONCLUSION
Caution should be exercised when using tPA on emphysema patients, we must be careful about DAH. The documented therapy of FVIIa has demonstrated a sustained and immediate hemostatic effect in DAH, however, still only in a small number of patients.
Larger studies are warranted, taking into account the high mortality of the DAH syndrome.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Colin AA, Shafieian M, Andreansky M. Bronchoscopic instillation of activated recombinant factor VII to treat diffuse alveolar hemorrhage in a child. Pediatr Pulmonol. 2010. 45: 411-
2. Fouret PJ, Touboul JL, Mayaud CM, Akoun GM, Roland J. Pulmonary Kaposi’s sarcoma in patients with acquired immune deficiency syndrome: A clinicopathological study. Thora×. 1987. 42: 262-8
3. Heslet L, Nielsen JD, Nepper-Christensen S. Local pulmonary administration of factor VIIa (rFVIIa) in diffuse alveolar hemorrhage (DAH)-a review of a new treatment paradigm. Biologics. 2012. 6: 37-46
4. Ioachimescu OC, Stoller JK. Diffuse alveolar hemorrhage: Diagnosing it and finding the cause. Cleve Clin J Med. 2008. 75: 258-80
5. Iskandar SB, Kasasbeh ES, Mechleb BK, Garcia I, Jackson A, Fahrig S. Alveolar hemorrhage: An underdiagnosed complication of treatment with glycoprotein IIb/IIIa inhibitors. J Interv Cardiol. 2006. 19: 356-63
6. Jauch EC, Saver JL, Adams HP, Bruno A, Demaerschalk BM, Khatri P. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2013. 44: 870-947
7. Kalra S, Bell MR, Rihal CS. Alveolar hemorrhage as a complication of treatment with abciximab. Chest. 2001. 120: 126-31
8. Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. Hospitals: Analysis of hospital records. Ann Intern Med. 2011. 154: 516-22
9. Marder VJ, Sherry S. Thrombolytic therapy: Current status (1). N Engl J Med. 1988. 318: 1512-20
10. Nasser M, Cottin V. Alveolar hemorrhage in vasculitis (primary and secondary). Semin Respir Crit Care Med. 2018. 39: 482-93
11. Newsome BR, Morales JE. Diffuse alveolar hemorrhage. South Med J. 2011. 104: 269-74
12. Rabe C, Appenrodt B, Hoff C, Ewig S, Klehr HU, Sauerbruch T. Severe respiratory failure due to diffuse alveolar hemorrhage: Clinical characteristics and outcome of intensive care. J Crit Care. 2010. 25: 230-5
13. Schwarz MI, Fontenot AP. Drug-induced diffuse alveolar hemorrhage syndromes and vasculitis. Clin Chest Med. 2004. 25: 133-40
14. Yigla M, Sprecher E, Azzam Z, Guralnik L, Kapeliovich M, Krivoy N. Diffuse alveolar hemorrhage following thrombolytic therapy for acute myocardial infarction. Respiration. 2000. 67: 445-8