- Department of Internal Medicine, University of Florida College of Medicine, Gainesville, USA
- University of Central Florida College of Medicine, Orlando, USA
- Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, Florida, USA
- Department of Neurological Surgery, University at Buffalo School of Medicine, Buffalo, New York, USA
Correspondence Address:
Ricardo J. Komotar
Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, Florida, USA
DOI:10.4103/sni.sni_264_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Aaron J. Franke, William Paul Skelton IV, Lindsey E. Woody, Amade Bregy, Ashish H. Shah, Kunal Vakharia, Ricardo J. Komotar. Role of bevacizumab for treatment-refractory meningiomas: A systematic analysis and literature review. 13-Jul-2018;9:133
How to cite this URL: Aaron J. Franke, William Paul Skelton IV, Lindsey E. Woody, Amade Bregy, Ashish H. Shah, Kunal Vakharia, Ricardo J. Komotar. Role of bevacizumab for treatment-refractory meningiomas: A systematic analysis and literature review. 13-Jul-2018;9:133. Available from: http://surgicalneurologyint.com/surgicalint-articles/role-of-bevacizumab-for-treatment%e2%80%91refractory-meningiomas-a-systematic-analysis-and-literature-review/
Abstract
Background:Meningiomas are the most prevalent primary tumor of the central nervous system (CNS), and although the majority of these neoplasms are classified as benign, nearly one fourth of the lesions display an aggressive profile characterized by pleomorphic histology, high recurrence rates, and overall resistance to standard treatment. Despite the ubiquitous nature of these tumors, no adjuvant therapeutic regimen has been identified which effectively controls disease recurrence and progression after surgery and radiation, leading to a dismal prognosis in this patient population. The primary focus of this research study is, hence, to assess the recently emerging use of bevacizumab, an anti-angiogenic agent, in the treatment of meningiomas. This systematic literature review analyzes the efficacy and safety of therapeutic bevacizumab for treatment-refractory meningiomas.
Methods:A systematic PubMed search was conducted according to PRISMA guidelines to identify all relevant reports investigating the anti-angiogenic agent bevacizumab in the treatment of intracranial meningiomas. The reported parameters from pertinent retrospective reviews, prospective studies, and case studies were volumetric reduction, radiographic response, clinical stability, overall survival (OS), and progression free survival (PFS) measured at 6 and 12 months postinitiation of treatment. Complications were cataloged based on the range and severity of the therapy-related toxicities.
Results:A total of 11 articles, 5 retrospective series, 2 prospective trials, and 4 case reports, reporting on a total of 92 patients, were included in this review. The use of bevacizumab therapy for intracranial meningiomas demonstrated median overall PFS of 16.8 months (range: 6.5-22 months) and PFS-6 of 73% (range: 44%-93%).
Conclusions:Therapeutic bevacizumab, either alone or with combination chemotherapies, for select patient populations with recurrent or progressive meningiomas, offers a treatment option that confers improved overall progression-free survival. To assess OS parameters, larger randomized controlled trials assessing the use of anti-angiogenic agents for recurrent/progressive meningiomas are warranted.
Keywords: Avastin, bevacizumab, meningioma, outcomes, systematic analysis
INTRODUCTION
Meningiomas account for 35.8% of all brain tumors making them the most common primary tumor of the central nervous system (CNS).[
Surgery remains a mainstay of treatment for meningiomas that have either grown on sequential imaging or those that cause symptoms. Typically, surgery is performed with curative intent by aiming for a Simpson Grade 1 resection (gross total resection with excision of the dural tail and overlying invaded cranium). In the event that such gross total resection is not attainable, clinicians may opt for a subtotal resection and adjuvant radiotherapy. The decision to use adjuvant radiation therapy is based on the extent of resection and certain characteristics of the histological analysis of the tumor, and is generally performed as adjuvant therapy in cases of atypical and anaplastic lesions as well as inoperable yet progressive Grade I lesions.[
Clinical trials investigating various chemotherapeutic agents, immunotherapies, and hormonal agents for recurrent meningiomas have been unfruitful, offering finite survival benefit with substantial drug toxicity.[
MATERIALS AND METHODS
Study selection
Using the MeSH database system of PubMed, a systematic literature search was performed between the years 2003 and 2017 for all articles containing the terms meningioma and bevacizumab ((“Meningioma [Mesh]”) AND “Bevacizumab [Mesh]”) following the PRISMA guidelines [
Data extraction
The analysis conducted on the results from each of the studies was based on the treatment cohort, pathological tumor diagnosis, any form of pretreatment received prior to the bevacizumab therapy, specifics of the administration protocol, clinical and radiographic response, and any adverse effects experienced during the adjuvant therapy. However, two case reports detailed the response rates in meningiomas when bevacizumab was used to treat other primary tumors, one breast cancer and one vestibular schwannoma. Some of the parameters established to assess the efficacy of bevacizumab therapy were tumor volumetric reduction, achievement of a radiographic response, clinical patient stability, overall survival (OS), and progression free survival (PFS) measured at defined points in time after initiation of treatment (i.e. at 6/12 months). Data review and analysis was used to assess the safety parameters of the treatment by looking at the range and severity of reported adverse effects experienced in patients during therapy protocol. Of note, neither did all of the studies in this review assess treatment efficacy using identical end-point parameters nor did some of the reports include the etiology of toxicities experienced. Due to these discrepancies among data protocols, our analysis has certain limitations in reporting comparative results (see below). All patient and treatment data available from the studies were included in this literature review.
RESULTS
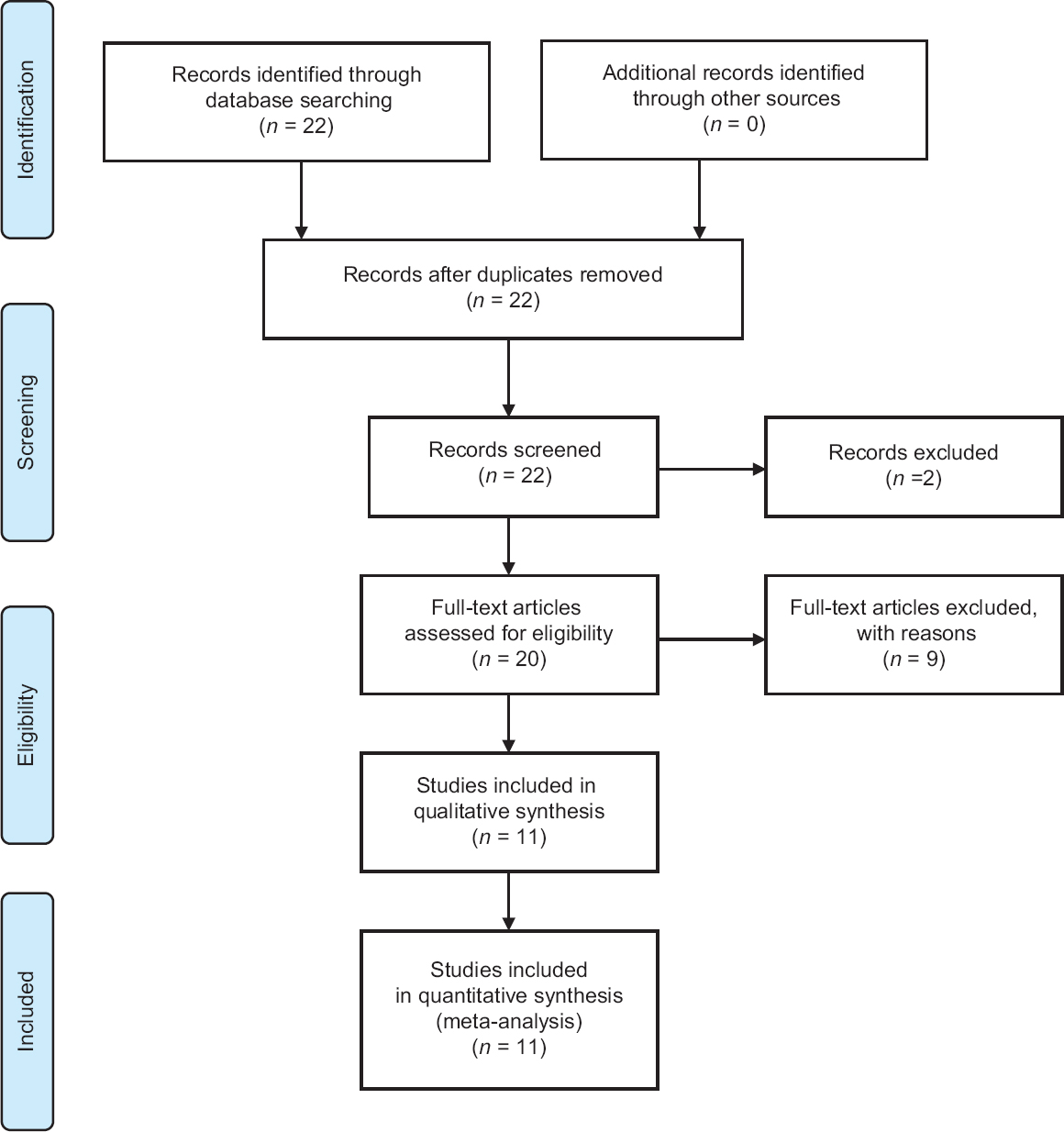
Study selection
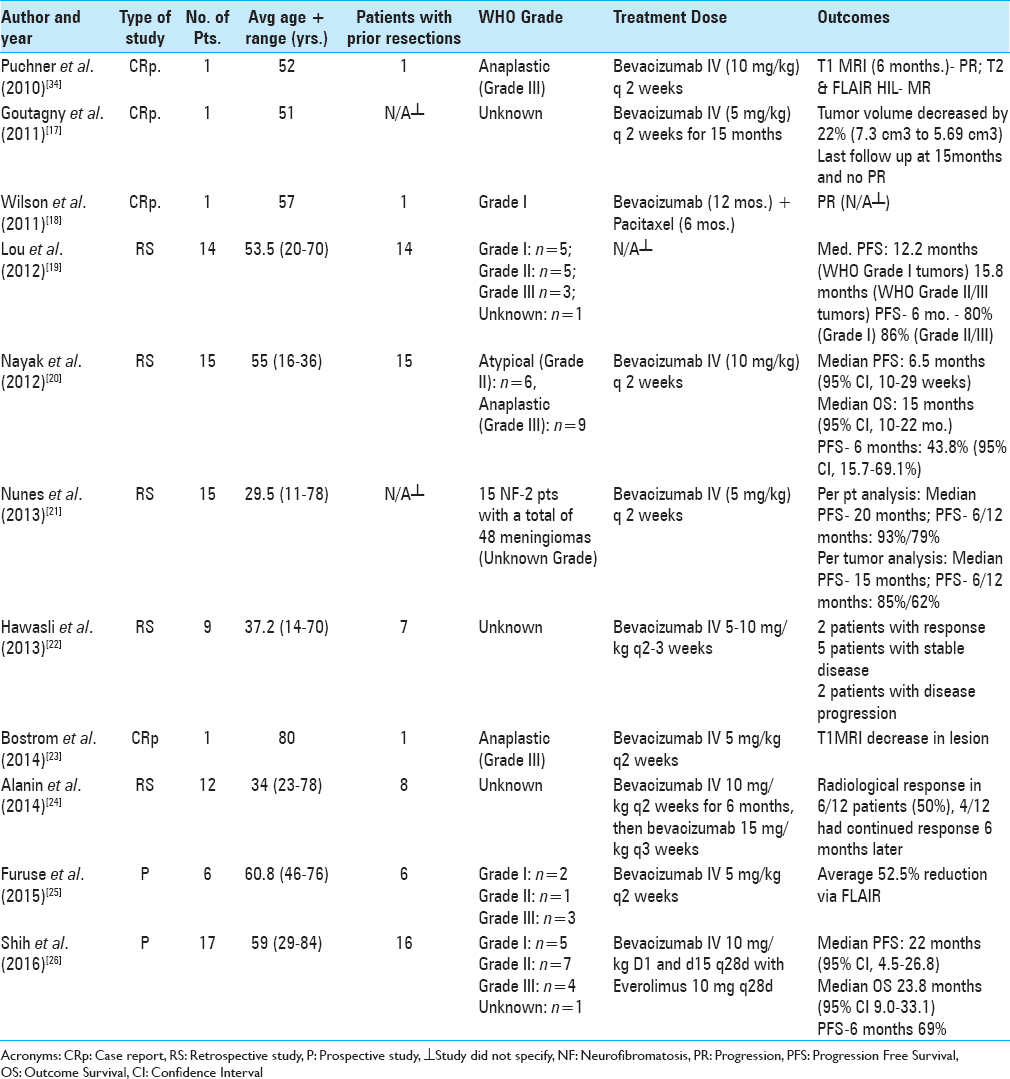
The preliminary literature search for studies using PubMed yielded 22 results. After selectively narrowing the criteria required of the studies, 11 articles and a total of 92 patients were included in our review. Five retrospective clinical trial series, two prospective trials, and four single patient case reports were included in this analysis. Patient demographics, specific diagnosis, pretreatment regimens, adjuvant drug therapy, and therapeutic bevacizumab doses and schedule of these studies are described in detail in
Pretreatment recurrences/treatments
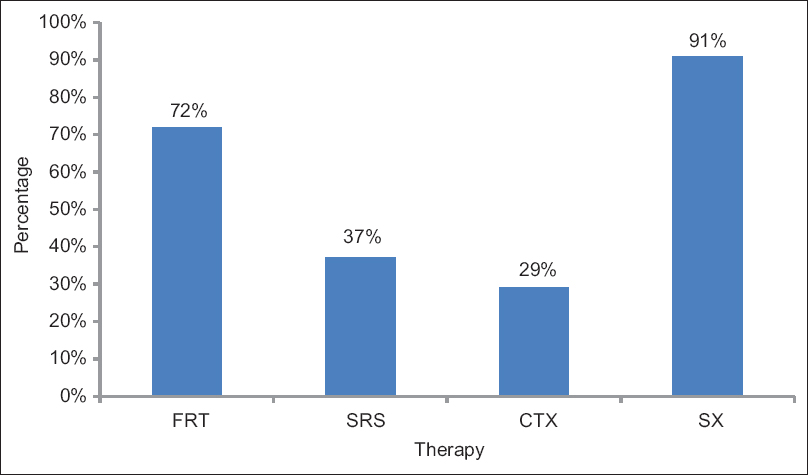
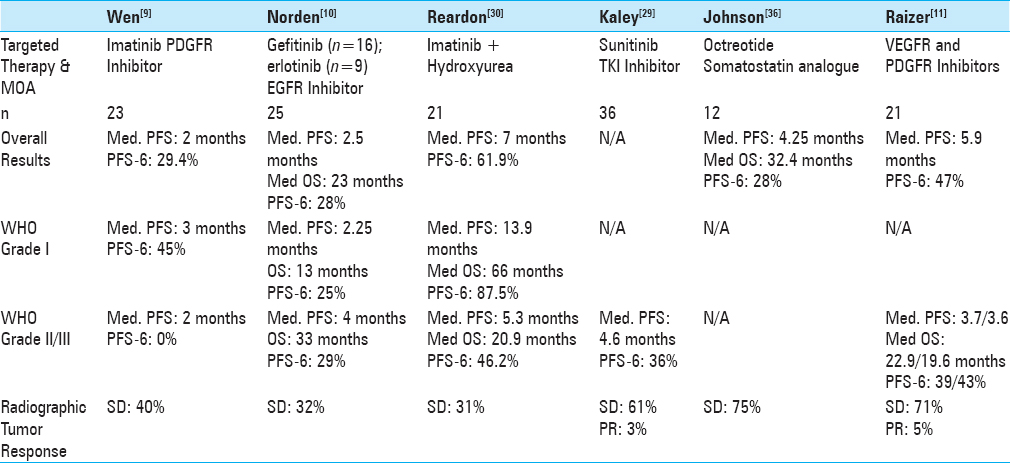
Nine of the studies, comprised of 76 patients, summarized in this review reported some form of prior meningioma treatment before starting patients on bevacizumab therapy. Among the participants in the studies retrieved, various combinations of pretreatment tumor management modalities were administered. The data show that 69 out of 76 (91%) patients from these nine studies underwent prior treatment with surgery. Of those, 30 patients underwent multiple surgeries for meningioma resection. Stereotactic radiosurgery was used in the pretreatment (pre-bevacizumab) regimen of 28 patients (37%). In all, 51 patients (72%) received fractionated RT prior to the start of bevacizumab; however, the dosing, regimen, and margins of RT varied among studies. Of note, 22 patients (29%) had received prior treatment with various chemotherapeutic and biological agents including imatinib mesylate (7), hydroxyurea (12), pasireotide (SOM230), a multi-ligand somatostatin receptor analogue (2), temozolomide (7), sunitinib (2), sandostatin (3), octreotide (3), tamoxifen (1), RU-486 (1), lovastatin (1), celecoxib (1), and etoposide (1) [
Bevacizumab dosage
Nine of the studies reported the administered dosage and schedule for the employed bevacizumab therapy. Bevacizumab was administered every 2 weeks, with four studies administering the drug at a dose of 5 mg/kg,[
Pathology
In evaluating the differences in tumor responses among patients, we must take into account the grade of the tumors treated and their susceptibility to anti-angiogenic therapy. In the case studies reported by Puchner et al. and Bostrom et al., the tumors had histological features of a grade III (anaplastic) meningioma, whereas the case report by Wilson et al. reported a grade I tumor. The retrospective series of Lou et al. included 14 patients: five (36%) with grade I meningioma, five (36%) had grade II (atypical) meningioma, three (21%) had grade III (anaplastic) meningioma, and one patient had a confirmed histologic diagnosis of meningioma, but of unspecified grade. The series identified by Nayak et al. reported 15 patients: six (40%) had grade II (atypical) meningioma and nine (60%) had grade III (anaplastic) meningioma. The prospective trial by Furuse et al. included six patients: two with grade I meningioma, one with grade II (atypical) meningioma, and three with anaplastic (grade III) meningioma. Finally, the phase II prospective trial by Shih et al. included 17 patients: five with grade I meningioma, seven with grade II meningioma, and four with grade III meningioma.
Survival
Using Kaplan–Meier survival curves, PFS and OS were documented for the patient cohorts, with both endpoints measured from the initiation of bevacizumab therapy. PFS was defined as the time from induction of therapy until death, initial disease progression, or last follow-up, assuming the patient remained alive without disease progression. OS was defined as the time from the therapy start date until death. Results analyzed from the studies (61 patients) demonstrated median overall PFS of 16.8 months (range: 6.5-22 months) and that 73% of patients were progression free at 6 months (PFS-6) (range: 44%-93%).
Bevacizumab safety
Overall, the majority of patients tolerated the dosage of bevacizumab used in these series with minimal complications. Evaluating the data reported for complication rates for the 92 patients included in this pooled cohort; grade III events were reported in 9.8% of patients, grade IV events in 4.3%, and two patients (2.1%) had a grade V event, of which the causality is uncertain in one. When comparing the dosages of bevacizumab (5 mg/kg vs. 10 mg/kg), there were no statistically significant differences in the frequencies of side effects between the two groups.
DISCUSSION
Although meningiomas are the most common primary CNS tumor, there is a paucity of data to support the use of most adjuvant chemotherapies, and no effective medical treatment currently exists for recurrent tumors after resection and radiation. Among the available FDA approved drugs is bevacizumab, a monoclonal antibody that binds to VEGF receptors forming a complex that inhibits binding of ligands and subsequently blocks angiogenesis.[
Five studies, with a total of 40 patients, in this review evaluated the tumors’ best radiographic response using the RANO (Response Assessment in Neuro-Oncology) criteria.[
The other six studies, with a total of 52 patients, used a percentage of volumetric change as measurement criteria for radiographic response. Radiographic response was defined as volumetric regression (decrease ≥20%), progression (increase ≥20%), or stable disease as any volumetric change in between. Regression analysis showed 12 patients (23%) had a radiographic response. In all, 26 patients (50%) had a stable disease, while 12 patients (23%) progressed. Two patients (4%) died prior to repeat imaging evaluation, and no patients had complete response.
Nayak et al. observed six patients with magnetic resonance imaging (MRI) findings of decreased T2 hyperintensities around the tumor, consistent with a reduction in peritumoral edema. After six weeks of therapy, FLAIR and T2-weighted MRI data reported by Puchner et al. exhibited regression in the hyper-intense lesions; both in the peritumoral edema zone and the center of the tumor. These observed radiographic responses were maintained at follow-up, 6 months after treatment cessation.
The Nayak review recorded the OS with a median OS of 15 months (95% CI: 10, 22 months). Due to the higher incidence of multifocal disease in patients with NF-2, Nunes et al. analyzed their data on a per-tumor as well as per-patient basis. The reported rates for the per-tumor PFS-6 and PFS-12 were 85% and 62%, respectively. The rates calculated for the per-patient PFS-6 and PFS-12 analyses were 93% and 79%, respectively, which accounts for multiple intracranial abnormalities. Lou and colleagues further analyzed the data and dichotomized the results based on WHO tumor grade and therapeutic treatment arms. The patient cohort with grade I meningiomas had a median PFS and PFS-6 of 12.2 months (95% CI: 1.1, 27.2) and 80% (95% CI: 20.4, 96.9), respectively, and 15.8 months (95% CI: 5.5, 17.9) and 87.5% (95% CI: 38.7, 98.1) for patients with grade II/III meningiomas, respectively. The four patients receiving treatment with bevacizumab as a single-agent therapy had a median PFS and PFS-6 of 15.8 months (95% CI: 12.2, ∞) and 100%, respectively, and 17.9 (95% CI: 1.1, 27.2) and 80% (95% CI: 40.9, 94.6), respectively, for the combination of bevacizumab plus chemotherapy group (n = 10). The Shih et al. phase II study was the only other study which showed OS. The median OS was 23.8 months (95% CI: 9.0-33.1), with median PFS of 22 months (95% CI: 4.5-26.8). PFS-6, 12, and 18-month rates were 69%, 57%, and 57%, respectively.
Most patients tolerated bevacizumab well, and the observed adverse effects were similar in type, severity, and incidence to those reported in patients treated with bevacizumab for glioblastoma (GBM).[
Potential uses of bevacizumab for meningiomas
The use of bevacizumab for patients with recurrent meningiomas is a matter of debate. However, its use may be most beneficial in special circumstances such as the ones listed below:
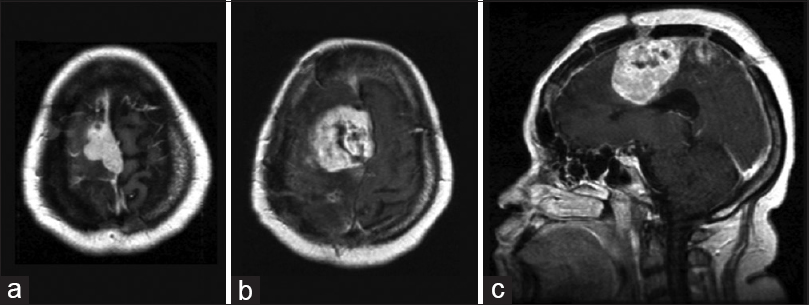
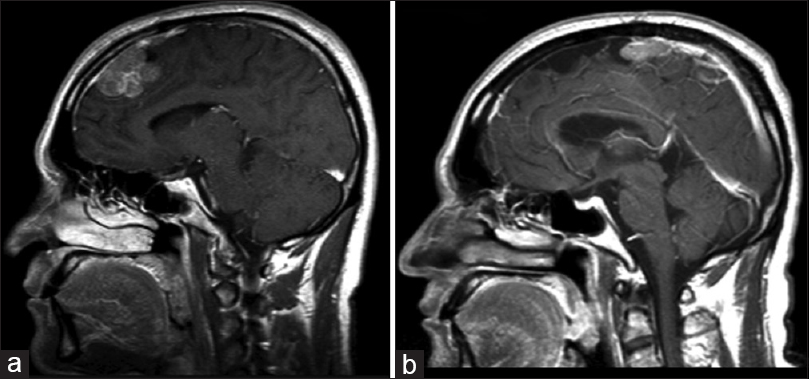
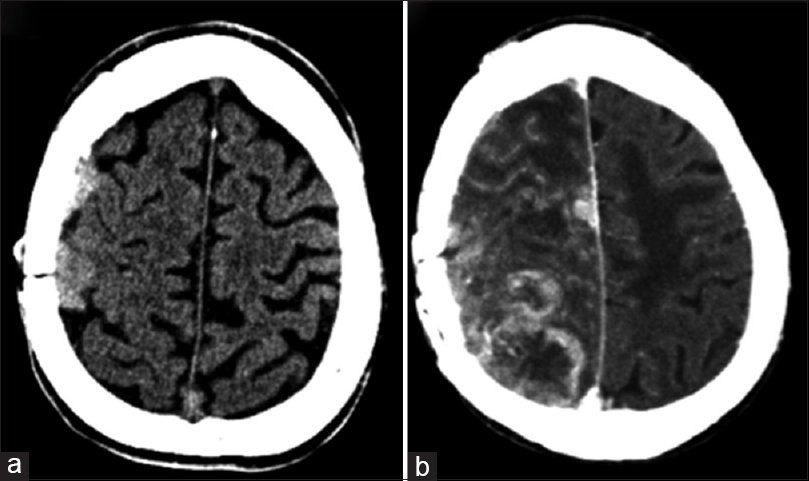
Treatment refractory/high-grade/high-vascularity cases In certain cases where treatment fails to prevent recurrence or progression, we advocate for the use of an adjuvant therapeutic agent such as bevacizumab. Although surgery and radiosurgery may be potential options for relapsing meningiomas, bevacizumab may delay recurrence and may also serve as a potential neoadjuvant option to radiation/surgery. Case illustration First patient is a 40-year-old female with a history of progressive left-sided hemiparesis and nausea and vomiting who presented to our institution with a large parasagittal meningioma extending over the central sulcus. Patient received a subtotal resection and adjuvant radiation therapy. After 5 years, tumor progression was noted in the surgical site, and a new lesion was noted along the anterior falx [ Case illustration Second patient is a 46-year-old male with a history of resection of an anterior parasagittal meningioma, who presented with progressive headaches 6 months later. Imaging revealed a large anterior frontal parasagittal meningioma along the anterior two thirds of the superior sagittal sinus. Patient was taken for surgery and a Simpson grade 3 resection was achieved. Histology at that time revealed a WHO grade III anaplastic meningioma. Over the next year, serial imaging revealed a left convexity meningioma [ Multiple meningiomas Patients with symptomatic multiple meningiomas are often difficult to treat due to the complexity of surgery, distance between lesions, and predisposition to recur. Resection is typically limited to the largest most symptomatic lesion and to surgically accessible lesions; therefore, adjuvant treatment such as radiation treatment (external beam RT or radiosurgery) or chemotherapy (bevacizumab) may be potential options for patients with meningiomas that continue to grow. Case illustration The patient is a 66-year-old female who presented with a lower cranial nerve palsies and left-sided hearing loss. MRI imaging revealed a large petroclival meningioma that continued to increase in size. The patient was taken for left suboccipital craniotomy (translabyrinthine approach), in which a subtotal resection was achieved. Subsequently, the patient underwent gamma knife radiosurgery, which failed to achieve control tumor. Two years later, the patient underwent another resection of residual tumor, again considered to be a gross total resection. However, the patient began to experience left-side radicular thoracic pain five years after the initial diagnosis. Subsequent imaging revealed multiple intradural extramedullary lesions in the thoracic spine from T3-T5 and T9-T10. Radiation-induced meningiomas Case illustration The patient is a 47-year-old male with a history of an intraparenchymal low-grade glioma (treated with gross total resection and RT 25 years prior) who presented with a new-onset seizure. MRI revealed a right frontal convexity radiation-induced meningioma; the patient subsequently had the surgery and a gross total resection was achieved. However, the tumor recurrence was noted after 2 years, and the patient was taken again for surgery [
Study limitations
As with all review studies, certain limitations exist that must be taken into account when interpreting pooled results. To begin with, the conclusions drawn from the individual series are restricted due to the small number of patients enrolled and the retrospective nature of the reports. Another finding limiting our analysis is the use of RANO criteria, which analyzes both enhancing and nonenhancing radiographic components, to assess tumor response to therapy. This response assessment paradigm was created to measure changes in malignant gliomas, not meningiomas, as was the case in our studies. An additional constraint in the interpretation of these studies comes from the fact that many patients received radiation and/or stereotactic radiosurgery prior to initiating bevacizumab therapy. This may have induced radionecrosis, which radiographically is indistinguishable from tumor progression. Furthermore, there was variation among the individual studies with respect to the dosage of bevacizumab administered, as well as differences in latency and duration of therapy. Finally, this review includes varying meningioma pathologies, some of which may grow at different rates and may require longer follow-up period to fully understand the pathophysiological changes observed. This may result in response rates that are blurred across various pathologies.
Future directions
The overall findings of these studies, even in the absence of survival parameters in some, offer encouraging results which warrant further investigations via large, prospective randomized control trials [
The observation that advanced meningiomas, grades II and III, has been associated with increased VEGF expression and hypervascularity,[
The molecular hypothesis guiding the rationale for using bevacizumab, and agents with comparable mechanisms of action, is based on the notion that VEGF as a key mediator of angiogenesis and edema formation has been shown to be expressed in 67% to 84% of meningiomas.[
Currently, there are several clinical trials underway using therapeutic agents targeting VEGF-directed pathways in patients with recurrent/progressive meningiomas. Notably, the multi-institutional phase II trial evaluating the combination therapy of bevacizumab and optune delivered electric field therapy (NCT02847559) and the phase II trial using bevacizumab as a single-agent therapy (NCT01125046), the former of which is still recruiting.
Further studies are required to identify medical therapies for patients who have progressed on avastin therapy. It is unclear whether these patients will benefit from continued avastin therapy or whether such therapy should be discontinued. KPS scores should also be included in these studies to further allow to ascertain the benefit of such therapies in the patient subgroups.
CONCLUSION
Patients with recurrent meningiomas currently have limited treatment options once they experience either recurrence or progression following the maximal surgical treatment and adjuvant radiation therapy. Given the overall morbidity with recurrent tumors and the subsequent poor survival, there is a critical need for effective therapies for these patients. The studies in this review, though limited by their small size and retrospective nature, offer encouraging efficacy and safety results with the use of bevacizumab, either alone or with combination chemotherapies, for selected patients. The data from these studies to date warrant further investigation in larger, prospective, randomized control trials evaluating the use of anti-angiogenic agents for recurrent/progressive meningiomas.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alanin MC, Klausen C, Caye-Thomasen P, Thomsen C, Fugleholm K, Poulsgaard L. The effect of bevacizumab on vestibular schwannoma tumour size and hearing in patients with neurofibromatosis type 2. Eur Arch Otorhinolaryngol. 2015. 272: 3627-33
2. Alexiou GA, Gogou P, Markoula S, Kyritsis AP. Management of meningiomas. Clin Neurol Neurosurg. 2010. 112: 177-82
3. Barresi V, Tuccari G. Evaluation of neo-angiogenesis in a case of chordoid meningioma. J Neurooncol. 2009. 95: 445-
4. Boström PDJ, Seifert M, Greschus S, Schäfer N, Glas M, Lammering G. Bevacizumab treatment in malignant meningioma with additional radiation necrosis. Strahlenther Onkol. 2014. 190: 416-21
5. Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995. 26: 86-91
6. Chamberlain MC, Glantz MJ. Interferon-α for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer. 2008. 113: 2146-51
7. Chamberlain MC, Glantz MJ, Fadul CE. Recurrent meningioma Salvage therapy with long-acting somatostatin analogue. Neurology. 2007. 69: 969-73
8. Chamberlain MC, Johnston SK. Hydroxyurea for recurrent surgery and radiation refractory meningioma: A retrospective case series. J Neurooncol. 2011. 104: 765-71
9. Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J Neurooncol. 2006. 78: 271-6
10. Chamberlain MC, Tsao-Wei DD, Groshen S. Temozolomide for treatment-resistant recurrent meningioma. Neurology. 2004. 62: 1210-2
11. Ding Y-S, Wang H-D, Tang K, Hu Z-G, Jin W, Yan W. Expression of vascular endothelial growth factor in human meningiomas and peritumoral brain areas. Ann Clin Lab Sci. 2008. 38: 344-51
12. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009. 27: 4733-40
13. Furuse M, Nonoguchi N, Kawabata S, Miyata T, Toho T, Kuroiwa T. Intratumoral and peritumoral post-irradiation changes, but not viable tumor tissue, may respond to bevacizumab in previously irradiated meningiomas. Radiat Oncol. 2015. 10: 156-
14. Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ. A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: A Southwest Oncology Group study. J Neurooncol. 1993. 15: 75-7
15. Goutagny S, Raymond E, Sterkers O, Colombani J, Kalamarides M. Radiographic regression of cranial meningioma in a NF2 patient treated by bevacizumab. Ann Oncol. 2011. 22: 990-1
16. Grunberg S, Rankin C, Townsend J, Ahmadi J, Feun L, Fredericks R. Phase III double-blind randomized placebo-controlled study of mifepristone (RU) for the treatment of unresectable meningioma. Proc Am Soc Clin Oncol. 2001. p.
17. Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, Grabenbauer GG. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: Results of a pilot study. J Neurooncol. 2005. 74: 157-65
18. Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: Diagnosis, characteristics, and treatment. J Neurooncol. 2010. 99: 433-43
19. Hawasli AH, Rubin JB, Tran DD, Adkins DR, Waheed S, Hullar TE. Antiangiogenic agents for nonmalignant brain tumors. J Neurol Surg Part B Skull Base. 2013. 74: 136-41
20. Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W. Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro oncol. 2011. 13: 530-5
21. Kaley TJ, Wen PY, Schiff D, Karimi S, DeAngelis LM, Nolan CP. Phase II trial of sunitinib (SU011248) for recurrent meningioma. Neuro Oncol. 2015. 17: 116-21
22. Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2008. 27: 740-5
23. Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ. Bevacizumab therapy for adults with recurrent/progressive meningioma: A retrospective series. J Neuro Oncol. 2012. 109: 63-70
24. Louis DN, Ohgaki H, Wiestler O, Cavenee W.editors. WHO classification of tumours of the central nervous system. 4. Lyon: IARC; 2007. p.
25. Loven D, Hardoff R, Bar Sever Z, Steinmetz AP, Gornish M, Rappaport ZH. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neuro Oncol. 2004. 67: 221-6
26. Marty M, Pivot X. The potential of anti-vascular endothelial growth factor therapy in metastatic breast cancer: Clinical experience with anti-angiogenic agents, focusing on bevacizumab. Eur J Cancer. 2008. 44: 912-20
27. Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningiomas. J Neurosurg. 2002. 97: 341-6
28. Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J. Atypical and anaplastic meningiomas treated with bevacizumab. J Neuro Oncol. 2012. 109: 187-93
29. Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neuro Oncol. 2010. 96: 211-7
30. Nunes FP, Merker VL, Jennings D, Caruso PA, di Tomaso E, Muzikansky A. Bevacizumab treatment for meningiomas in NF2: A retrospective analysis of 15 patients. PLoS One. 2013. 8: e59941-
31. Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neurooncology. 2013. 15: ii1-ii56
32. Pistolesi S, Boldrini L, Gisfredi S, Ieso KD, Camacci T, Caniglia M. Angiogenesis in intracranial meningiomas: Immunohistochemical and molecular study. Neuropathol Appl Neurobiol. 2004. 30: 118-25
33. Provias J, Claffey K, Lau N, Feldkamp M, Guha A. Meningiomas: Role of vascular endothelial growth factor/vascular permeability factor in angiogenesis and peritumoral edema. Neurosurgery. 1997. 40: 1016-26
34. Puchner M, Hans V, Harati A, Lohmann F, Glas M, Herrlinger U. Bevacizumab-induced regression of anaplastic meningioma. Ann Oncol. 2010. 21: 2445-6
35. Raizer JJ, Grimm SA, Rademaker A, Chandler JP, Muro K, Helenowski I. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. Journal of neuro-oncology. 2014. 117: 93-101
36. Reardon DA, Norden AD, Desjardins A, Vredenburgh JJ, Herndon JE, Coan A. Phase II study of Gleevec® plus hydroxyurea (HU) in adults with progressive or recurrent meningioma. J Neuro Oncol. 2012. 106: 409-15
37. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neuro Oncol. 2010. 99: 393-405
38. Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997. 86: 840-4
39. Shih KC, Chowdhary S, Rosenblatt P, Weir AB, Shepard GC, Williams JT. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neuro Oncol. 2016. 129: 281-8
40. Wen PY, Quant E, Drappatz J, Beroukhim R, Norden AD. Medical therapies for meningiomas. J Neuro Oncol. 2010. 99: 365-78
41. Wen PY, Yung WA, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE. Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01-08). Neurooncology. 2009. 11: 853-60
42. Wilson TJ, Heth JA. Regression of a meningioma during paclitaxel and bevacizumab therapy for breast cancer. J Clin Neurosci. 2012. 19: 468-9