- Department of Neurosurgery, College of Medical Sciences, Bharatpur, Nepal
Correspondence Address:
Sunil Munakomi
Department of Neurosurgery, College of Medical Sciences, Bharatpur, Nepal
DOI:10.4103/2152-7806.177125
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sunil Munakomi, Binod Bhattarai, Balaji Srinivas, Iype Cherian. Role of computed tomography scores and findings to predict early death in patients with traumatic brain injury: A reappraisal in a major tertiary care hospital in Nepal. 19-Feb-2016;7:23
How to cite this URL: Sunil Munakomi, Binod Bhattarai, Balaji Srinivas, Iype Cherian. Role of computed tomography scores and findings to predict early death in patients with traumatic brain injury: A reappraisal in a major tertiary care hospital in Nepal. 19-Feb-2016;7:23. Available from: http://surgicalneurologyint.com/surgicalint_articles/role-computed-tomography-scores-findings-predict-early-death-patients-traumatic-brain-injury-reappraisal-major-tertiary-care-hospital-nepal/
Abstract
Background:Glasgow Coma Scale has been a long sought model to classify patients with head injury. However, the major limitation of the score is its assessment in the patients who are either sedated or under the influence of drugs or intubated for airway protection. The rational approach for prognostication of such patients is the utility of scoring system based on the morphological criteria based on radiological imaging. Among the current armamentarium, a scoring system based on computed tomography (CT) imaging holds the greatest promise in conquering our conquest for the same.
Methods:We included a total of 634 consecutive neurosurgical trauma patients in this series, who presented with mild-to-severe traumatic brain injury (TBI) from January 2013 to April 2014 at a tertiary care center in rural Nepal. All pertinent medical records (including all available imaging studies) were reviewed by the neurosurgical consultant and the radiologist on call. Patients’ worst CT image scores and their outcome at 30 days were assessed and recorded. We then assessed their independent performance in predicting the mortality and also tried to seek the individual variables that had significant interplay for determining the same.
Results:Both imaging score (Marshall) and clinical score (Rotterdam) can be used to reliably predict mortality in patients with acute TBI with high prognostic accuracy. Other specific CT characteristics that can be used to predict early mortality are traumatic subarachnoid hemorrhage, midline shift, and status of the peri-mesencephalic cisterns.
Conclusion:We demonstrated in this cohort that though the Marshall score has the high predictive power to determine the mortality, better discrimination could be sought through the application of the Rotterdam score that encompasses various individual CT parameters. We thereby recommend the use of such comprehensive prognostic model so as to augment our predictive power for properly dichotomizing the prognosis of the patients with TBI. In the future, it will therefore be important to develop prognostic models that are applicable for the majority of patients in the world they live in, and not just a privileged few who can use resources not necessarily representative of their societal environment.
Keywords: Computed tomography variables, Marshall score, prognosis, Rotterdam score, traumatic brain injury
INTRODUCTION
Traumatic brain injury (TBI) is a silent epidemic that has paramount short- and long-term consequences. To allow proper resource allocation (which is of preeminent importance in developing countries) there is a need for such system that correctly predicts the outcome. One of the most widely used clinical pearls for such prediction is the Glasgow Coma Scale (GCS).[
The introduction of the scoring model has revolutionized the treatment algorithm for the patients with head injuries with leaps and bounces.[
Previous studies have verified the role of CT scores in predicting the mortality of patients following TBI.[
In our context, the trauma-related injury is the third leading cause of overall deaths.[
MATERIALS AND METHODS
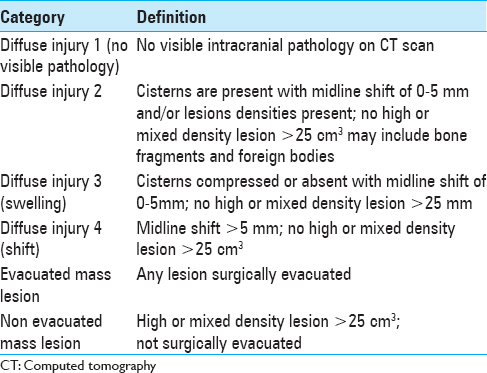
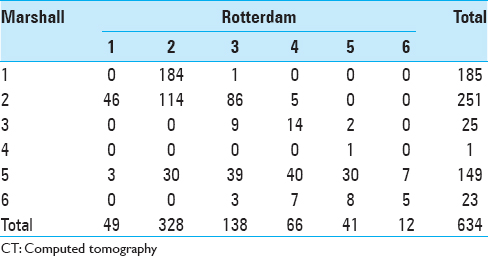
This study included 634 consecutive neurosurgical trauma patients with mild-to-severe TBI admitted in the Department of Neurosurgery, College of Medical Sciences, Bharatpur, Nepal, from January 2013 to August 2014. Data were collected prospectively, and each patient underwent a full clinical examination and imaging on admission. Both imaging score (Marshall) [
Factors such as hypotension, hypoxia, seizure, time lapse to arrival to hospital, and prehospital management do have an impact on the outcome of the patients. But in the context of a rural medical infrastructure, the proper prehospital management via a rapid response ambulance and paramedical team are still lacking. All the patients brought to the emergency with head injury were initially triaged according to the revised trauma score. After achieving hemodynamic stability, CT scanning and other relevant investigations were carried out to fully assess or rule out coexisting polytrauma as a confounding factor for mortality of the patient.
This study was approved by the ethics board of the institution. We avoided a purely retrospective study to avoid a post hoc effect on to the scoring system. Written consent was obtained from all patients or their relatives.
RESULTS
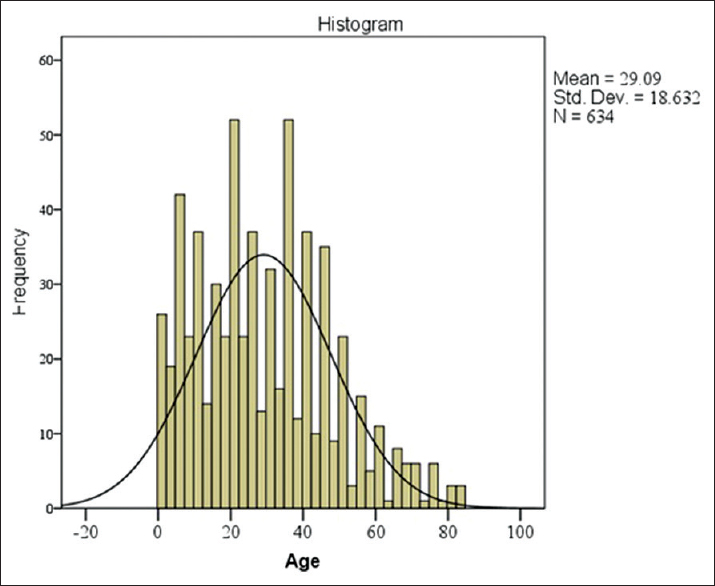
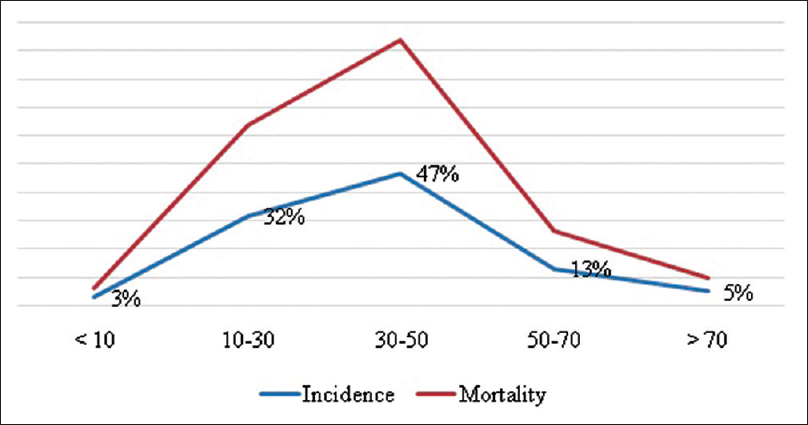
The mean age of the patient with a head injury was 29.1 years, and the age group in our cohort with the highest number of patients enrolled was at ages between 30 and 50 years [
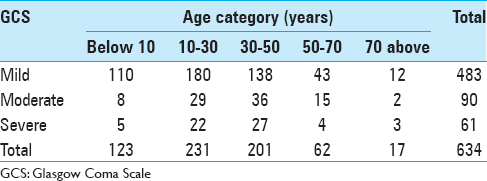
When the age group was adjusted for patients with moderate and severe head injury only, the number of patients was also highest in the age group of 30–50 years (47%), followed by the age group of 10–30 years (32%), the age group of 50–70 years (13%), the age group of above 70 (5%), and finally the age group below 10 years (3%). The mortality was highest in the age group of 30–50 years (46.66%) followed by age group of 10–30 years (31.66%), 50–70 years (13.33%), above 70 years (5%), and finally below 10 years (3.33%) [
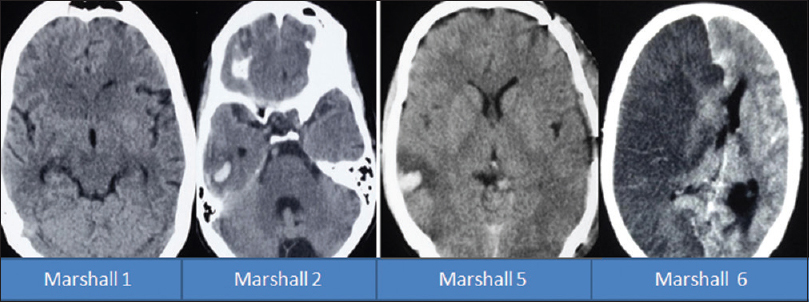
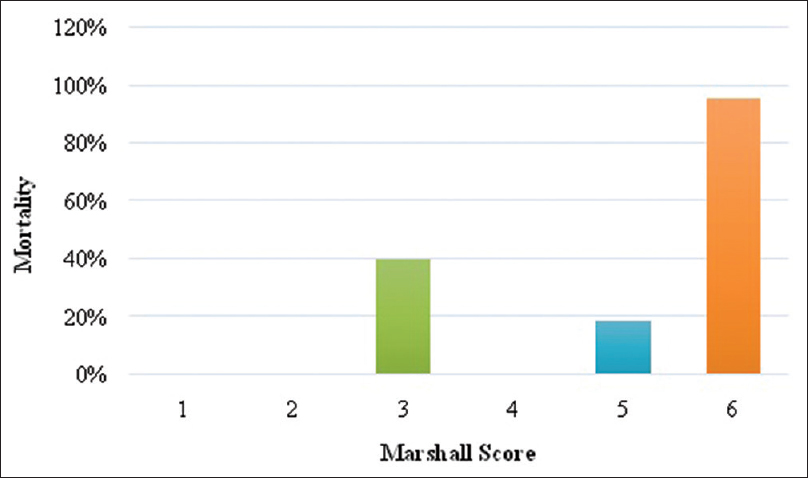
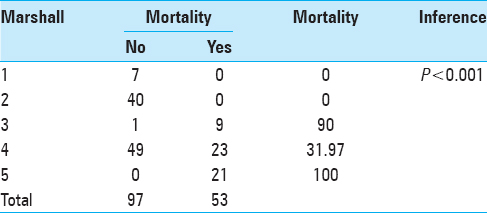
This table signifies the importance of the Marshall CT score in predicting mortality in patients with TBI. The mortality in patients with Marshall score 1 and 2 in our cohort was 0%, for score 3 it was 40%, for score 4 it was 0%, for score 5 it was 18.79%, and for score 6 was 95.66% [
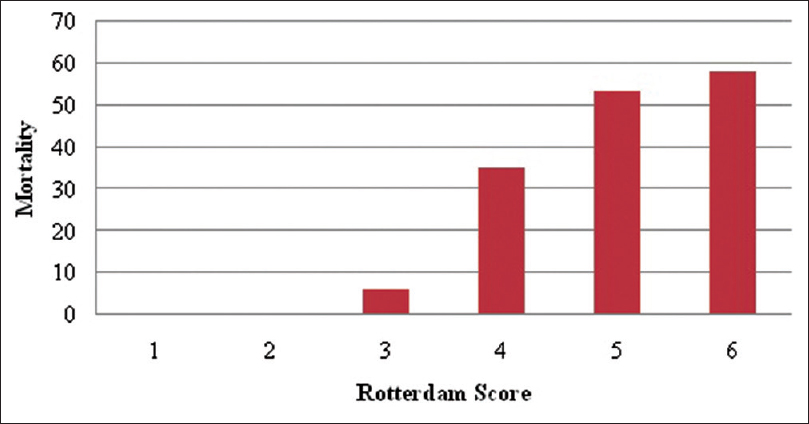
The mortality in patients with Rotterdam score 1 and 2 in our cohort was 0%, for score 3 it was 6%, for score 4 it was 35%, for score 5 it was 53.65%, and for score 6 it was 58.33% [
When the Marshall score is adjusted for the patients with moderate and severe head injury, then the mortality in patients with score 1 and 2 was 0%, for score 3 was 90%, for score 4 was 31.97%, for score 5 was 31.97%, and for score 6 was 100% [
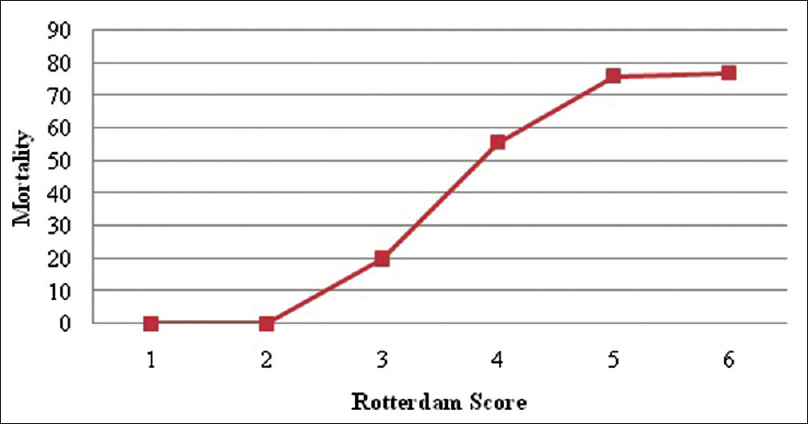
The Rotterdam score adjusted for patients with moderate and severe head injury shows that the mortality for the patients with scores of 1 and 2 was 0%, for score 3 was 20%, for score 4 was 55.85%, for score 5 was 76%, and for score 6 was 77% [
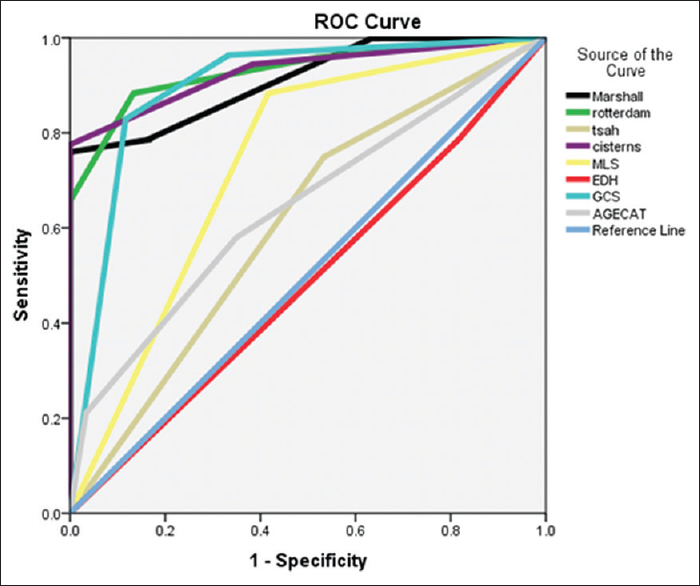
The area under the curve (AUC) was significant for both the scoring system with 0.912 for Marshall and 0.929 for Rotterdam scores, respectively. When the study included other variables, then the AUC was 0.929 for cisternal anatomy, 0.897 for GCS score, 0.733 for MLS, and 0.643 for age category, respectively, in predicting the mortality [
DISCUSSION
Our cohort study clearly corroborates the validity of Marshall and Rotterdam scores in its ability to predict early mortality in patients with acute TBI. It demonstrates the role of early surgical intervention in reducing the overall mortality. We are furthermore able to show that distinct individual parameters such as GCS, age, status of the cisterns, presence of MLS, and traumatic SAH had a significant impact on the outcome of these patients.
However, a number of limitations regarding the use of CT score models must be recognized.[
Another drawback of the CT scoring model is the issue of interobserver or interrater bias. Studies have shown that there is a significant difference in scoring of the CT image among trained radiologist as well.[
Proper dichotomization of the patients with a head injury has an immense impact on the outcome as well as the proper allocation of the available resources in developing countries like ours. Prognostication models should provide a mirror image of the life in the long run for both the patients and their caregivers. Dichotomizing into “good” or “poor” may though be helpful for the immediate care decisions, but it may be just covering the loopholes for correctly projecting the real scenario in the long-term.
SUMMARY AND CONCLUSION
This study concludes that the radiological Marshall CT score has a strong predictive power for predicting the outcome after TBI, but greater discrimination and more relevant interpretation can be ascertained with the application of clinical scores utilizing individual CT parameters as attempted in the Rotterdam scoring model. Consequently, for prognostication purposes, this study recommends the use of a set of specific imaging characteristics obtained in individuals rather than using broad categories derived from the existing CT classification systems alone. In the future, it will therefore be important to develop prognostic models that are applicable for the majority of patients in the world they live in, and not just a privileged few who can use resources not necessarily representative of their societal environment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Azian AA, Nurulazman AA, Shuaib L, Mahayidin M, Ariff AR, Naing NN. Computed tomography of the brain in predicting outcome of traumatic intracranial haemorrhage in Malaysian patients. Acta Neurochir (Wien). 2001. 143: 711-20
2. Bahloul M, Chelly H, Ben Hmida M, Ben Hamida C, Ksibi H, Kallel H. Prognosis of traumatic head injury in South Tunisia: A multivariate analysis of 437 cases. J Trauma. 2004. 57: 255-61
3. Bajracharya A, Agrawal A, Yam B, Agrawal C, Lewis O. Spectrum of surgical trauma and associated head injuries at a university hospital in Eastern Nepal. J Neurosci Rural Pract. 2010. 1: 2-8
4. Balestreri M, Czosnyka M, Chatfield DA, Steiner LA, Schmidt EA, Smielewski P. Predictive value of Glasgow coma scale after brain trauma: Change in trend over the past ten years. J Neurol Neurosurg Psychiatry. 2004. 75: 161-2
5. Bhandari R, Mahato IP, Poudel M, Giri R. Head injury in Nepal. Health Renaissance. 2010. 8: 110-3
6. Buechler CM, Blostein PA, Koestner A, Hurt K, Schaars M, McKernan J. Variation among trauma centers’ calculation of Glasgow coma scale score: Results of a national survey. J Trauma. 1998. 45: 429-32
7. Caroli M, Locatelli M, Campanella R, Balbi S, Martinelli F, Arienta C. Multiple intracranial lesions in head injury: Clinical considerations, prognostic factors, management, and results in 95 patients. Surg Neurol. 2001. 56: 82-8
8. Chesnut RM. Evolving models of neurotrauma critical care: An analysis and call to action. Clin Neurosurg. 2000. 46: 185-95
9. Cordobés F, Lobato RD, Rivas JJ, Cabrera A, Sarabia M, Castro S. Post-traumatic diffuse axonal brain injury. Analysis of 78 patients studied with computed tomography. Acta Neurochir (Wien). 1986. 81: 27-35
10. Eisenberg HM, Gary HE, Aldrich EF, Saydjari C, Turner B, Foulkes MA. Initial CT findings in 753 patients with severe head injury. A report from the NIH traumatic coma data bank. J Neurosurg. 1990. 73: 688-98
11. Firsching R, Woischneck D, Klein S, Reissberg S, Döhring W, Peters B. Classification of severe head injury based on magnetic resonance imaging. Acta Neurochir (Wien). 2001. 143: 263-71
12. Greene KA, Jacobowitz R, Marciano FF, Johnson BA, Spetzler RF, Harrington TR. Impact of traumatic subarachnoid hemorrhage on outcome in nonpenetrating head injury. Part II: Relationship to clinical course and outcome variables during acute hospitalization. J Trauma. 1996. 41: 964-71
13. Havill JH, Sleigh JW, Davis GM, Chatterton BJ, Gilbert KW, Marsh NV. Observer error and prediction of outcome - Grading of head injury based on computerised tomography. Crit Care Resusc. 2001. 3: 15-8
14. Hernández AV, Steyerberg EW, Butcher I, Mushkudiani N, Taylor GS, Murray GD. Adjustment for strong predictors of outcome in traumatic brain injury trials: 25% reduction in sample size requirements in the IMPACT study. J Neurotrauma. 2006. 23: 1295-303
15. Kakarieka A, Braakman R, Schakel EH. Classification of head injuries based on computerized tomography: Prognostic value. Neurologia. 1995. 10: 159-61
16. Kakarieka A, Braakman R, Schakel EH. Clinical significance of the finding of subarachnoid blood on CT scan after head injury. Acta Neurochir (Wien). 1994. 129: 1-5
17. Liu HM, Tu YK, Su CT. Changes of brainstem and perimesencephalic cistern: Dynamic predictor of outcome in severe head injury. J Trauma. 1995. 38: 330-3
18. Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005. 57: 1173-82
19. Maas AI, Steyerberg EW, Butcher I, Dammers R, Lu J, Marmarou A. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: Results from the IMPACT study. J Neurotrauma. 2007. 24: 303-14
20. Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN. The mayo classification system for traumatic brain injury severity. J Neurotrauma. 2007. 24: 1417-24
21. Mardjono T, Muhammad ZA, Arwinder SG, Ahmad F. Early mortality predictor of severe traumatic brain injury: A single center study of prognostic variables based on admission characteristics. Indian J Neurotrauma. 2013. 10: 3-8
22. Mattioli C, Beretta L, Gerevini S, Veglia F, Citerio G, Cormio M. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: A multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J Neurosurg. 2003. 98: 37-42
23. Moskopp D, Stähle C, Wassmann H. Problems of the Glasgow coma scale with early intubated patients. Neurosurg Rev. 1995. 18: 253-7
24. Narayan RK, Greenberg RP, Miller JD, Enas GG, Choi SC, Kishore PR. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg. 1981. 54: 751-62
25. Okten AI, Gezercan Y, Ergün R. Traumatic subarachnoid hemorrhage: A prospective study of 58 cases. Ulus Travma Acil Cerrahi Derg. 2006. 12: 107-14
26. Ono J, Yamaura A, Kubota M, Okimura Y, Isobe K. Outcome prediction in severe head injury: Analyses of clinical prognostic factors. J Clin Neurosci. 2001. 8: 120-3
27. Pillai SV, Kolluri VR, Praharaj SS. Outcome prediction model for severe diffuse brain injuries: Development and evaluation. Neurol India. 2003. 51: 345-9
28. Quattrocchi KB, Prasad P, Willits NH, Wagner FC. Quantification of midline shift as a predictor of poor outcome following head injury. Surg Neurol. 1991. 35: 183-8
29. Selladurai BM, Jayakumar R, Tan YY, Low HC. Outcome prediction in early management of severe head injury: An experience in Malaysia. Br J Neurosurg. 1992. 6: 549-57
30. Servadei F, Murray GD, Penny K, Teasdale GM, Dearden M, Iannotti F. The value of the “worst” computed tomographic scan in clinical studies of moderate and severe head injury. European brain injury consortium. Neurosurgery. 2000. 46: 70-5
31. Servadei F, Murray GD, Teasdale GM, Dearden M, Iannotti F, Lapierre F. Traumatic subarachnoid hemorrhage: Demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002. 50: 261-7
32. Servadei F, Nasi MT, Giuliani G, Cremonini AM, Cenni P, Zappi D. CT prognostic factors in acute subdural haematomas: The value of the ‘worst’ CT scan. Br J Neurosurg. 2000. 14: 110-6
33. Shrestha A, Joshi RM, Thapa A, Devkota UP, Gongal DN. Outcome of traumatic brain injury in head injury patients undergoing surgical management: A tertiary level neuro-centre experience. Kathmandu Univ Med J. 2011. 9: 283-5
34. Siegel S, Castellan NJ.editorsNonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1988. p.
35. Teasdale G, Galbraith S, Murray L, Ward P, Gentleman D, McKean M. Management of traumatic intracranial haematoma. Br Med J (Clin Res Ed). 1982. 285: 1695-7
36. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974. 2: 81-4
37. Uchino Y, Okimura Y, Tanaka M, Saeki N, Yamaura A. Computed tomography and magnetic resonance imaging of mild head injury - Is it appropriate to classify patients with Glasgow coma scale score of 13 to 15 as “mild injury”?. Acta Neurochir (Wien). 2001. 143: 1031-7
38. van Dongen KJ, Braakman R, Gelpke GJ. The prognostic value of computerized tomography in comatose head-injured patients. J Neurosurg. 1983. 59: 951-7
39. Young B, Rapp RP, Norton JA, Haack D, Tibbs PA, Bean JR. Early prediction of outcome in head-injured patients. J Neurosurg. 1981. 54: 300-3