- Department of Neurosurgery, Nara Medical University, Nara, Japan
Correspondence Address:
Ichiro Nakagawa
Department of Neurosurgery, Nara Medical University, Nara, Japan
DOI:10.4103/2152-7806.196377
Copyright: © 2016 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Akiko Marutani, Ichiro Nakagawa, Hun Soo Park, Kentaro Tamura, Yasushi Motoyama, Hiroyuki Nakase. Ruptured aneurysm at the cortical segment of the distal posterior inferior cerebellar artery associated with hemodynamic stress after basilar artery occlusion. 21-Dec-2016;7:
How to cite this URL: Akiko Marutani, Ichiro Nakagawa, Hun Soo Park, Kentaro Tamura, Yasushi Motoyama, Hiroyuki Nakase. Ruptured aneurysm at the cortical segment of the distal posterior inferior cerebellar artery associated with hemodynamic stress after basilar artery occlusion. 21-Dec-2016;7:. Available from: http://surgicalneurologyint.com/surgicalint_articles/ruptured-aneurysm-at-the-cortical-segment-of-the-distal-posterior-inferior-cerebellar-artery-associated-with-hemodynamic-stress-after-basilar-artery-occlusion/
Abstract
Background:A distal posterior inferior cerebellar artery (PICA) de novo aneurysm at the cortical segment after atherosclerotic basilar artery occlusion is extremely rare. Here, we report the case of a ruptured distal PICA de novo aneurysm 8 years after basilar artery occlusion.
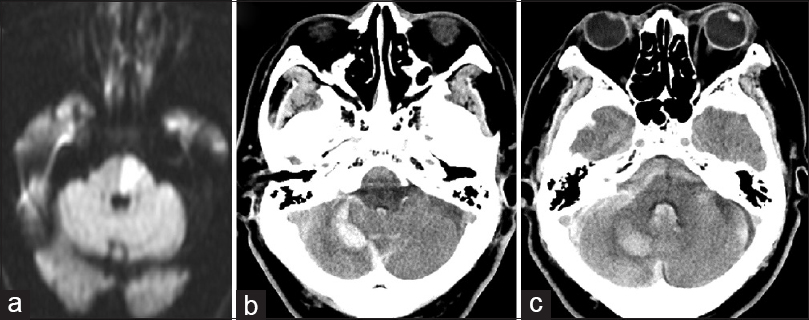
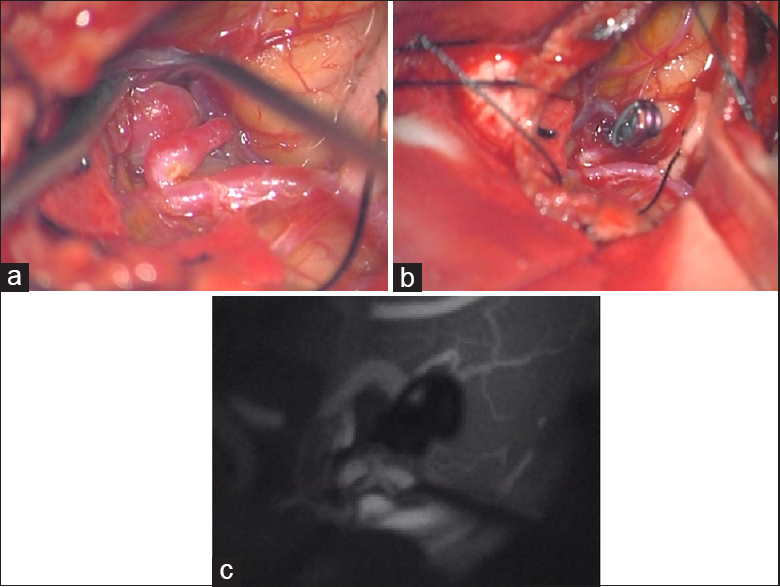
Case Description:A 75-year-old man experienced sudden disturbance of consciousness; computed tomography demonstrated cerebellar and subarachnoid hemorrhage due to a ruptured distal PICA aneurysm. Neck clipping of the aneurysm prevented re-rupture initially, and superficial temporal artery-superior cerebellar artery (STA-SCA) bypass was performed 3 months after admission. Postoperative angiography confirmed patency of the bypass, and the patient was discharged without any new neurological deficits.
Conclusion:This report describes a case of de novo development of a saccular distal PICA aneurysm after atherosclerotic basilar artery occlusion. We believe that increased hemodynamic stress at the PICA might have contributed to the occurrence and rupture of the aneurysm. STA-SCA bypass, introduced in the territory of the cerebellar hemisphere, reduces hemodynamic stress, which would prevent the occurrence of de novo aneurysm and recurrent bleeding.
Keywords: Distal posterior inferior cerebellar artery aneurysm, hemodynamic stress, neck clipping, superficial temporal artery-superior cerebellar artery bypass
INTRODUCTION
Posterior inferior cerebellar artery (PICA) aneurysms account for 0.49–3.0% of all intracranial aneurysms.[
CASE REPORT
A 75-year-old man had a history of left brainstem infarction due to atherosclerotic BA occlusion at the age of 67 years [
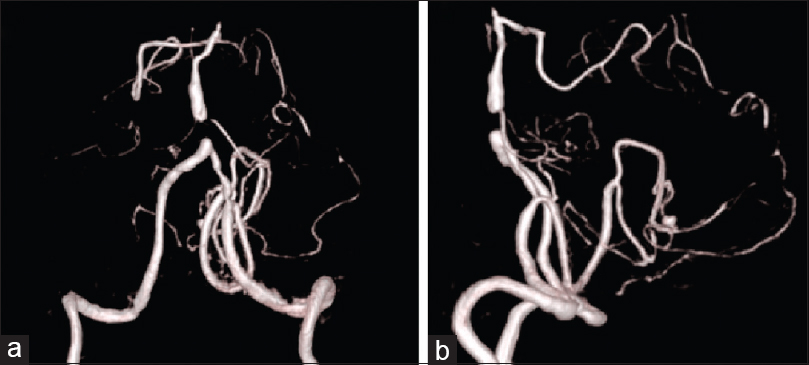
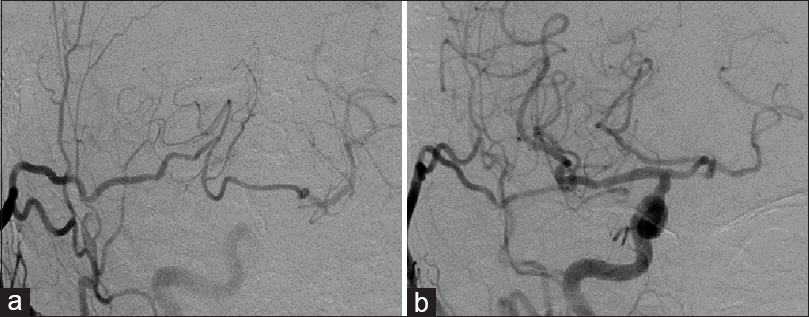
One week after admission to our hospital, three-dimensional (3D) computed tomography angiography (CTA) and digital subtraction angiography (DSA) revealed a 3.6-mm distal PICA saccular aneurysm at the cortical segment [
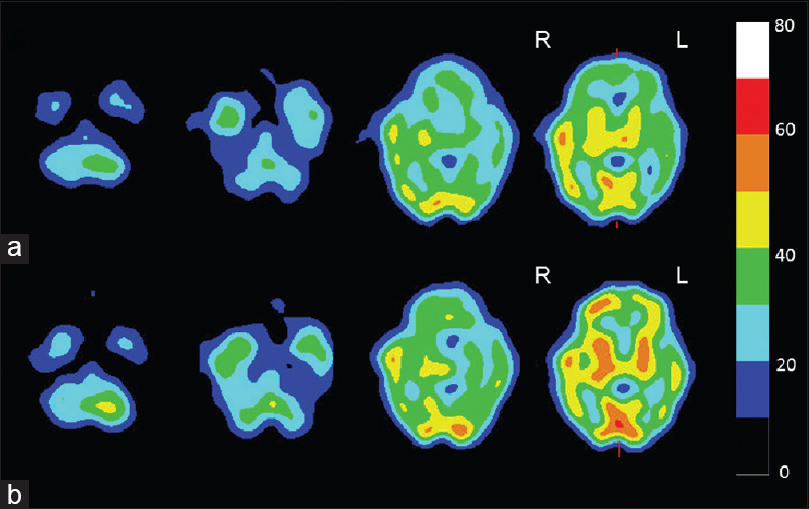
A week after the neck clipping, brain scintigraphy showed cerebellar hemodynamic ischemia (right dominant side). Hemodynamic cerebral ischemia can be stratified into Stage I and II ischemia (Misery perfusion). According to vasodilatory and metabolic compensation for reduction of cerebral perfusion pressure, stage II ischemia is defined as a reduction of the resting cerebral blood flow (<34 mL/100 g/min), loss of vascular reserve (<10%), and elevation of the oxygen extraction fraction. Accordingly, in the Japan Extracranial-Intracranial Bypass Trial,[
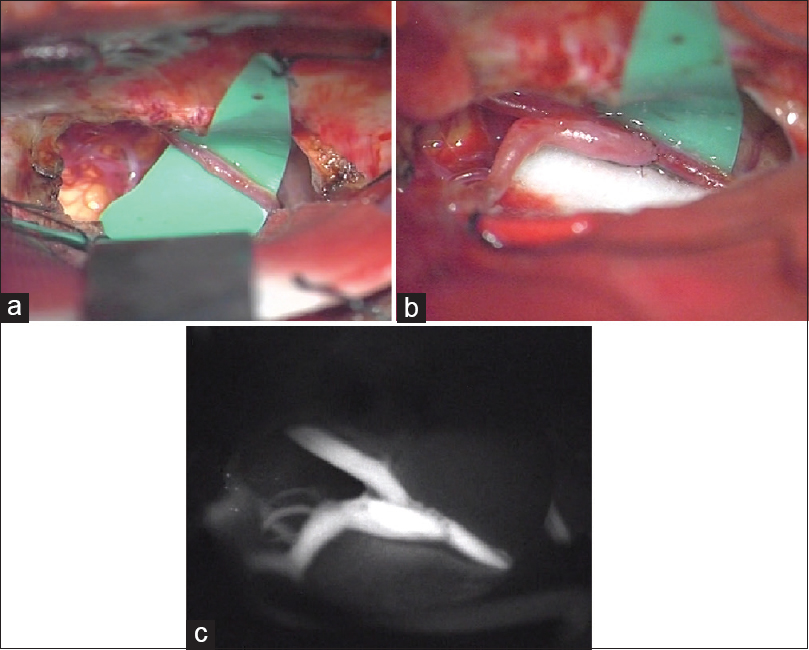
Three months after the onset of subarachnoid hemorrhage (SAH), right STA-SCA bypass was performed via the right subtemporal approach in the “parkbench” position to prevent the occurrence of a hemodynamic stress-induced de novo aneurysm and recurrent bleeding [
Figure 5
Postoperative digital subtraction angiography performed a month after bypass surgery. Right external cerebral angiography showing the establishment of new blood vessels from the right superior cerebellar artery to the left superior cerebellar artery (a), and right common carotid angiography showing bilateral posterior cerebral arteries via the posterior communicating artery (b)
Written informed consent was obtained from the patient for the publication of this report.
DISCUSSION
We reported the case of a patient with a ruptured distal PICA aneurysm at the cortical segment 8 years after occlusion of the BA. We performed STA-SCA bypass after neck clipping to reduce hemodynamic stress and avoid recurrence of the PICA aneurysm. To the best of our knowledge, this is the first case report of hemodynamic stress reduction with STA-SCA bypass after neck clipping to avoid recurrence of a PICA aneurysm.
Since the first case of a ruptured aneurysm in the PICA was reported in 1991,[
The identification of the aneurysm on MRI may be difficult because of its small size. A previous study reported that the PICA aneurysm size was generally small (2–3 mm in diameter) and that angiography facilitated aneurysm detection at this rare location.[
CONCLUSION
We reported a case of a distal PICA de novo aneurysm in the cortical segment, which induced hemodynamic stress in the PICA because of basilar artery occlusion. Increased hemodynamic stress may result in a vessel-induced aneurysm, and STA-SCA bypass can reduce hemodynamic stress, which might prevent recurrent cerebellar bleeding and the occurrence of a de novo PICA aneurysm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Amin-Hanjani S, Goodin S, Charbel FT, Alaraj A. Resolution of bilateral moyamoya associated collateral vessel aneurysms: Rationale for endovascular versus surgical intervention. Surg Neurol Int. 2014. 5: S155-60
2. Bertalanffy H, Schmidek HH, Roberts DW.editors. Surgical management of aneurysms of the vertebral and posterior inferior cerebellar artery complex. Schmidek and Sweet's Operative Neurosurgical Technique Indications, Methods, and Results. Philadelphia: Saunders Elsevier; 2006. p. 1209-23
3. Chen W, Xing W, Peng Y, He Z, Wang C, Wang Q. Diagnosis and treatment of intracranial aneurysms using 320-detector row volumetric computed tomography angiography. World Neurosurg. 2016. 91: 347-56
4. Hlavin ML, Takaoka Y, Smith AS. A “PICA communicating artery” aneurysm: Case report. Neurosurgery. 1991. 29: 926-9
5. Horie N, Takahashi N, Furuichi S, Mori K, Onizuka M, Tsutsumi K. Ruptured aneurysm at the choroidal branch of the posterior inferior cerebellar artery: A case report and review of the literature. Surg Neurol. 2003. 60: 540-4
6. Horiuchi T, Tanaka Y, Hongo K, Nitta J, Kusano Y, Kobayashi S. Characteristics of distal posteroinferior cerebellar artery aneurysms. Neurosurgery. 2003. 53: 589-95
7. JET Study Group. Japanese EC-IC Bypass Trial (JET Study): The second interim analysis. Surg Cereb Stroke. 2002. 30: 434-7
8. Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: A long-term follow-up study. Stroke. 2001. 32: 485-91
9. Lister JR, Rhoton AL, Matsushima T, Peace DA. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982. 10: 170-99
10. Matsumoto J, Uemura S, Hayasaki A, Kimura H, Morioka M, Kuratsu J. Ruptured aneurysm at an anastomotic artery extending from the vertebral artery to the posterior inferior cerebellar artery: A case report. Acta Neurochir. 2011. 153: 931-5
11. Miyamoto S, Yoshimoto T, Hashimoto N, Okada Y, Tsuji I, Tominaga T. Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: Results of the Japan Adult Moyamoya Trial. Stroke. 2014. 45: 1415-21
12. Sugiyama S, Fujimura M, Inoue T, Shimizu H, Watanabe M, Tominaga T. Ruptured aneurysm of a posterior inferior cerebellar artery communicating artery. Neurol Med Chir. 2012. 52: 81-3
13. Tokimura H, Yamahata H, Kamezawa T, Tajitsu K, Nagayama T, Sugata S. Clinical presentation and treatment of distal posterior inferior cerebellar artery aneurysm. Neurosurg Rev. 2011. 34: 57-67