- Department of Neurousurgery, Japanese Red Cross Fukui Hospital, Fukui,
- Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto,
- Department of Otorhinolaryngology, Japanese Red Cross Fukui Hospital, Fukui, Japan,
- Department of Pathology, Japanese Red Cross Fukui Hospital, Fukui, Japan.
Correspondence Address:
Noritaka Sano, Department of Neurosurgery, Kyoto University Graduate School of Medicine, Kyoto, Japan.
DOI:10.25259/SNI_567_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Noritaka Sano1,2, Hiroyuki Ikeda1, Yoshitaka Tsujimoto1, Makoto Hayase1, Sadaharu Torikoshi1, Taiyo Morikawa3, Tadakazu Okoshi4, Masaki Nishimura1, Hiroki Toda1. Ruptured fungal mycotic internal carotid artery aneurysm successfully treated with stent-assisted coil embolization: A case report. 02-Sep-2022;13:392
How to cite this URL: Noritaka Sano1,2, Hiroyuki Ikeda1, Yoshitaka Tsujimoto1, Makoto Hayase1, Sadaharu Torikoshi1, Taiyo Morikawa3, Tadakazu Okoshi4, Masaki Nishimura1, Hiroki Toda1. Ruptured fungal mycotic internal carotid artery aneurysm successfully treated with stent-assisted coil embolization: A case report. 02-Sep-2022;13:392. Available from: https://surgicalneurologyint.com/surgicalint-articles/11850/
Abstract
Background: Ruptured intracranial fungal mycotic aneurysms have a high mortality rate. It has been reported that the number of opportunistic infections has increased. Here, we report the first case of a patient in which a ruptured fungal carotid artery aneurysm was successfully treated by stent-assisted coil embolization.
Case Description: A 76-year-old male receiving dual antiplatelet therapy due to a recent percutaneous transluminal angioplasty presented with blurred vision of the right eye and diplopia. Magnetic resonance imaging revealed a fungal mass in the sphenoid sinus, and the patient was pathologically diagnosed with invasive aspergillosis. After receiving oral voriconazole for 4 weeks, he was admitted to the hospital with hemorrhagic shock from epistaxis. The right internal carotid artery angiography revealed a de novo irregularly shaped aneurysm at the cavernous portion, projecting into the sphenoid sinus, which was considered to be the source of bleeding. Due to the lack of ischemic tolerance and urgent demand for hemostasis, we performed a stent-assisted coil embolization of the aneurysm without interrupting the blood flow. Postoperatively, the patient had no neurological deficit, and treatment with voriconazole was continued for 12 months without rebleeding.
Conclusion: Stent-assisted coil embolization without parent artery occlusion might be a promising option for the urgent treatment of ruptured fungal mycotic aneurysms. Long-term administration of voriconazole might be continued for 12 months for such patients.
Keywords: Aspergillus, Coil embolization, Fungal aneurysm, Mycotic aneurysm, Stent
INTRODUCTION
Invasive aspergillosis can cause a number of problems, including meningitis, brain abscess, orbital apex syndrome, or rarely, mycotic aneurysms, especially in immunocompromised patients.[
CASE DESCRIPTION
History
A 76-year-old right-handed male with a history of diabetes mellitus, hypertension, and coronary artery disease suffered from repetitive transient ischemic attacks due to severe progressive stenosis, which had been diagnosed 5 years before. He underwent a percutaneous transluminal angioplasty and stenting with Wingspan 4.5 mm*15 mm (Stryker, MI, U.S.A.) of a cavernous-petrous portion of the right ICA [
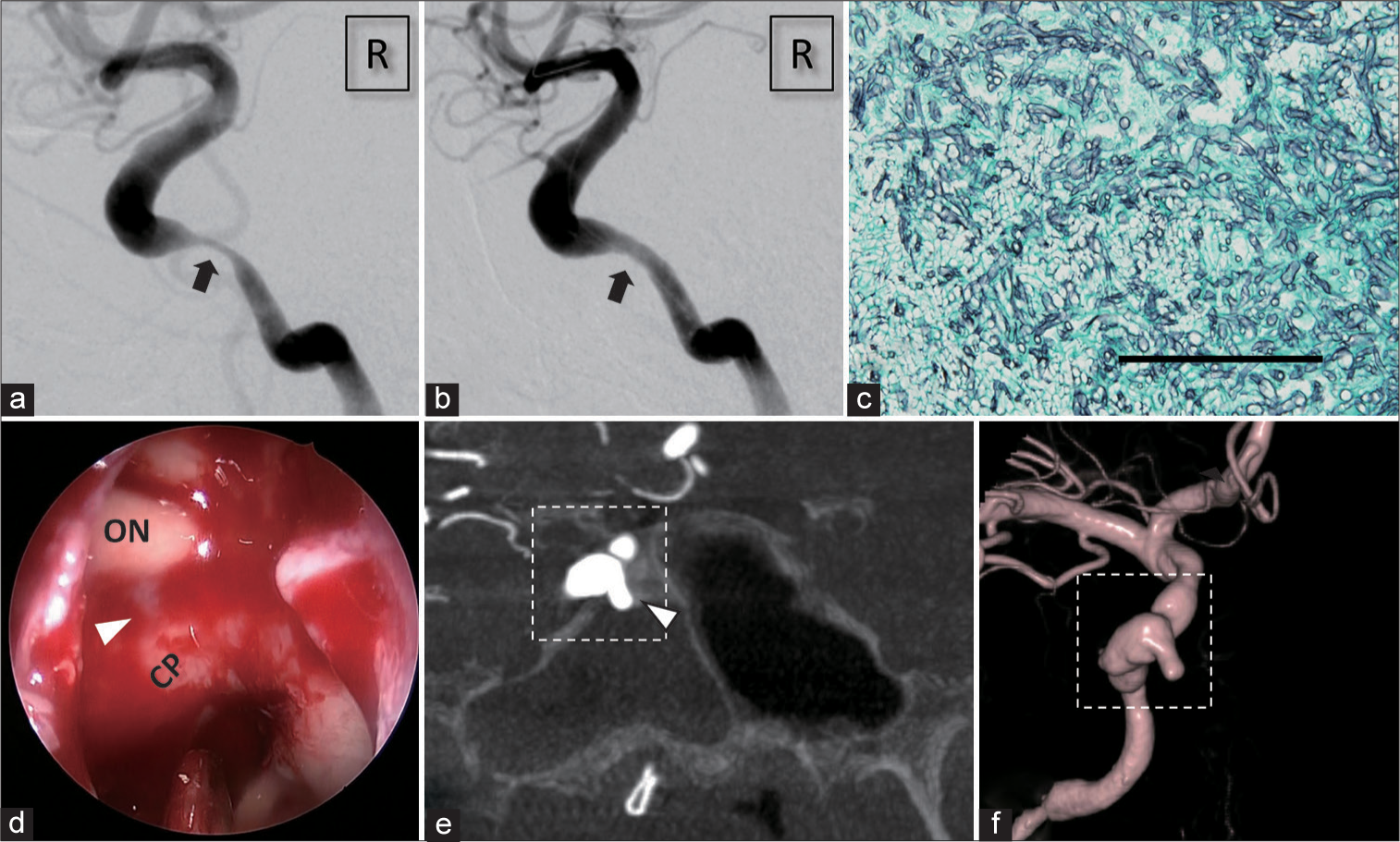
Figure 1:
Imaging findings before the embolization. (a and b) The right internal carotid artery (ICA) angiography before and after the percutaneous transluminal angioplasty and stent deployment of the petrous ICA stenosis (arrow). (c) Grocott (modified Gomori methenamine silver) stain of the fungus ball in the sphenoid sinus showing branching septate hyphae typical of Aspergillus species (original magnification ×200, black bar = 100 um). (d) Endoscopic view in the sphenoid sinus during the hemostatic procedure. A pulsatile projection (arrowhead) between ON and CP was seen while no active bleeding was confirmed (ON: optic nerve and CP: carotid prominence). (e) Coronal section of the right three-dimensional rotational angiography. A tip of irregularly shaped aneurysm projected into the right sphenoid sinus (arrowhead), and the sinus was filled with blood. (f) Three-dimensional rotational angiography showing irregularly shaped stenosis and dilatation. Dotted rectangles in (e) and (f) indicate the same portion of the aneurysm.
A fiber endoscopy revealed that the source of bleeding was from the sphenoid sinus. Tentative hemostasis was achieved by a posterior tamponade with hemostatic balloon and gauze. However, soon after the hemostatic instrument had been removed, he suffered from massive epistaxis. A blood transfusion and urgent hemostasis were required. A retrospective review of the endoscopic hemostasis procedure revealed pulsatile projection on the roof of the sphenoid sinus [
Endovascular procedure
To achieve urgent hemostasis, we chose stent-assisted coil embolization to preserve the blood flow of the ICA. First, a microcatheter for coil embolization was navigated into the aneurysm. Then, we deployed LVIS BLUE 4.0 mm*28 mm (Terumo, Tokyo, JAPAN) in the ICA from just proximal to the posterior communicating artery. The coverage of the stent was increased at the protruding part, to the cavernous portion overlapping partially into the Wingspan stent. After that, we packed the aneurysm with a coil using the jailing technique [
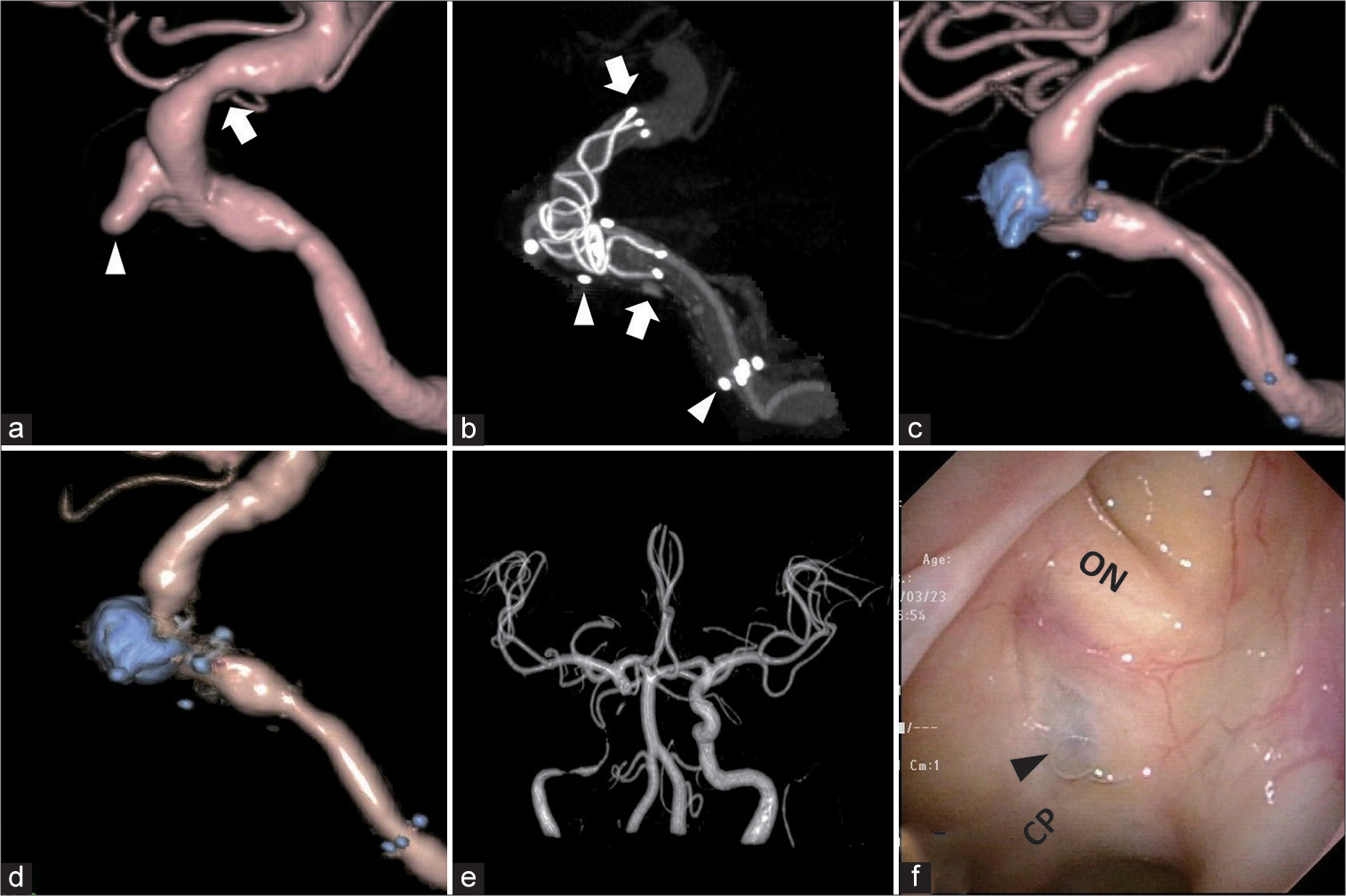
Figure 2:
Imaging findings during and after the embolization. (a) The right internal carotid artery (ICA) three-dimensional rotational angiography, lateral view. There were de novo aneurysmal projection (arrowhead) and stenosis (arrow), which were not seen 8 weeks before [Figure 1a and b]. (b) Three-dimensional rotational angiography after the deployment of the LVIS BLUE stent. The stent was deployed between the arrows and the proximal part was overlapped with the Wingspan stent (between the arrowheads). (c) The right ICA angiography after embolization showing no blood flow in the aneurysm and slight improvement of stenosis indicated in (a). (d) The right ICA angiography 6 months after embolization. There was no relapse of the aneurysm, while slight in-stent restenosis was seen in the Wingspan stent. (e) Magnetic resonance angiography 12 months after the embolization. There was no de novo intracranial aneurysm. Blood flow around the coils and stents cannot be seen because of the magnetic substance effect. (f) Endoscopic view in the sphenoid sinus 12 months after the embolization. Coils in the aneurysm were seen through the mucous membrane in the sphenoid sinus (arrowhead).
Postoperative course
Immediately following the procedure, his blurred vision and diplopia completely resolved. Subsequently, he continued oral voriconazole, and his serum β-d-Glucan levels returned to a normal level within 3 months. The patient never experienced epistaxis or neurological deterioration postoperatively. Furthermore, we regularly conducted magnetic resonance angiography (MRA) and digital subtraction angiography in every 3 months, and there was no recurrence or the appearance of the de novo aneurysm in the downstream of the right ICA. Moreover, three-dimensional rotational ICA angiography revealed no recurrence of the aneurysm after 6 months [
DISCUSSION
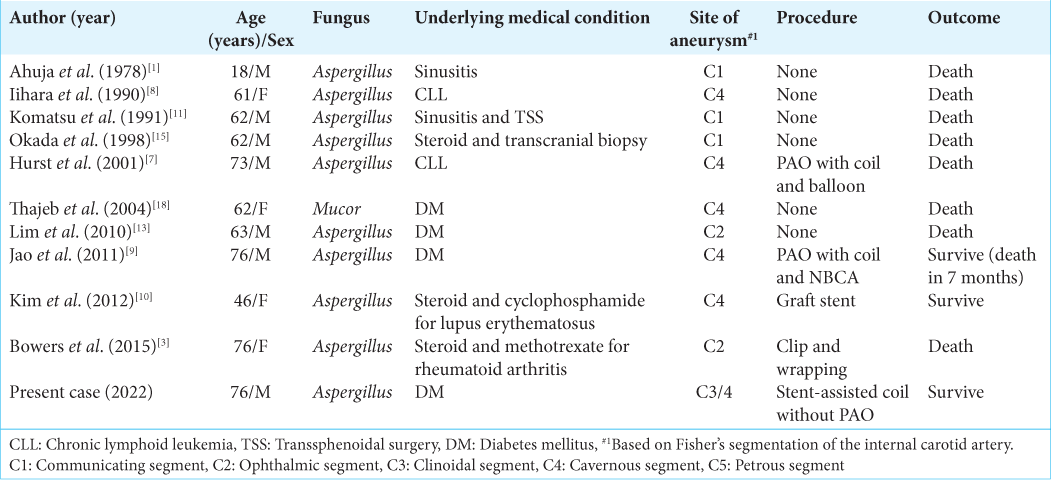
To the best of our knowledge, this is the first report of a fungal mycotic intracranial ICA aneurysm preserving blood flow in the parent artery and being successfully treated with stent-assisted coil embolization. There are few reports of fungal mycotic aneurysms of the ICA, and almost all cases are associated with an immunocompromised condition, including chemotherapy or diabetes mellitus.[
Ruptured intracranial fungal mycotic ICA aneurysms are generally fatal. Conservative treatment with antifungal drugs is not enough for hemostasis and resulted in death in all reported cases [
In our case, massive epistaxis due to the de novo aneurysm in cavernous ICA occurred only 8 weeks after the deployment of a Wingspan stent, and urgent cessation of the antiplatelet therapy would result in-stent thrombosis or occlusion. Direct surgery was considered less favorable due to the risk of bleeding from the patient being on dual antiplatelet therapy and the risk of ischemic complications if prompt parent artery occlusion without a maneuver to ensure collateral blood flow. Stent-assisted coil embolization of the aneurysm might be a good option, as it ensures the blood flow of the parent artery while blocking the inflow to the irregularly shaped aneurysm. There is the possibility of a rupture occurring in the fragile wall of the fungal mycotic aneurysm during the maneuver. However, we could stop the bleeding by occlusion of the aneurysm in the cavernous sinus with minimal access to the fragile bleb projecting into the sphenoid sinus. Moreover, even if the aneurysm ruptured into the cavernous sinus, it would not cause a fatal subarachnoid hemorrhage, but a direct carotid-cavernous fistula (CCF). Furthermore, CCF can be treated in the course of continuous endovascular embolization of cavernous sinus with coils. Despite this, an otolaryngologist was ready for direct hemostasis using fiber endoscopy in case of uncontrolled epistaxis during the procedure.
It can be challenging to select the antifungal agent to use and decide the duration of postoperative treatment. Voriconazole has become standard therapy for invasive aspergillosis involving the central nervous system,[
CONCLUSION
Invasive aspergillosis can directly affect the cavernous ICA and cause a fungal mycotic aneurysm. If the aneurysm ruptures, this can cause subarachnoid hemorrhage or massive epistaxis, which is often fatal. In such cases, urgent hemostasis is needed. In cases with poor collateral blood flow and an aneurysm localized in the cavernous-petrous portion, stent-assisted coil embolization might be an alternative option for the occlusion of the parent artery. Continuing voriconazole for 12 months might be a reasonable strategy after such procedure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We wish to thank Tadashi Sagou for the precise radiological diagnosis and Daisuke Uesaka for making high-resolution digital subtraction angiography.
References
1. Ahuja GK, Jain N, Vijayaraghavan M, Roy S. Cerebral mycotic aneurysm of fungal origin. Case report. J Neurosurg. 1978. 49: 107-10
2. Baeesa SS, Bakhaidar M, Ahamed NA, Madani TA. Invasive orbital apex aspergillosis with mycotic aneurysm formation and subarachnoid hemorrhage in immunocompetent patients. World Neurosurg. 2017. 102: 42-8
3. Bowers CA, Saad D, Clegg DO, Ng P, Clayton F, Haydoura S. Rapidly fatal internal carotid artery mycotic aneurysm rupture in a rheumatoid patient taking a TNF-α inhibitor: Case report and literature review. J Neurol Surgery A Cent Eur Neurosurg. 2015. 76: 249-54
4. Grosjean P, Weber R. Fungus balls of the paranasal sinuses: A review. Eur Arch Otorhinolaryngol. 2007. 262: 461-70
5. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002. 347: 408-15
6. Hot A, Mazighi M, Lecuit M, Poirée S, Viard JP, Loulergue P. Fungal internal carotid artery aneurysms: Successful embolization of an aspergillus-associated case and review. Clin Infect Dis. 2007. 45: e156-61
7. Hurst RW, Judkins A, Bolger W, Chu A, Loevner LA. Mycotic aneurysm and cerebral infarction resulting from fungal sinusitis: Imaging and pathologic correlation. Am J Neuroradiol. 2001. 22: 858-63
8. Iihara K, Makita Y, Nabeshima S, Tei T, Keyaki A, Nioka H. Aspergillosis causing of the central nervous system from mycotic aneurysm of the basilar artery. Neurol Med Chir. 1990. 30: 618-23
9. Jao SY, Weng HH, Wong HF, Wang WH, Tsai YH. Successful endovascular treatment of intractable epistaxis due to ruptured internal carotid artery pseudoaneurysm secondary to invasive fungal sinusitis. Head Neck. 2011. 33: 437-40
10. Kim YC, Lee H, Ryu HH, Beom SH, Yang Y, Kim S. Aspergillus-associated cerebral aneurysm successfully treated by endovascular and surgical intervention with voriconazole in lupus nephritis patient. J Korean Med Sci. 2012. 27: 317-20
11. Komatsu Y, Narushima K, Kobayashi E, Tomono Y, Nose T. Aspergillus mycotic aneurysm-case report. Neurol Med Chir (Tokyo). 1991. 31: 346-50
12. Kothary MH, Chase T, Macmillan JD. Correlation of elastase production by some strains of aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun. 1984. 43: 320-25
13. Lim SC, Choi JU, Bae SH. Rapid development of an infectious aneurysm of the internal carotid artery from orbital apex syndrome. Otolaryngol Head Neck Surg. 2010. 142: 294-5
14. Morriss FH, Spock A. Intracranial aneurysm secondary to mycotic orbital and sinus infection: Report of a case implicating penicillium as an opportunistic fungus. Am J Dis Child. 1970. 119: 357-62
15. Okada Y, Shima T, Nishida M, Yamane K, Yoshida A. Subarachnoid hemorrhage caused by aspergillus aneurysm as a complication of transcranial biopsy of an orbital apex lesion: Case report. Neurol Med Chir (Tokyo). 1998. 38: 432-7
16. Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005. 106: 2641-5
17. Schwartz S, Reisman A, Troke PF. The efficacy of voriconazole in the treatment of 192 fungal central nervous system infections: A retrospective analysis. Infection. 2011. 39: 201-10
18. Thajeb P, Thajeb T, Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scand J Infect Dis. 2004. 36: 643-8
19. Yamaguchi J, Kawabata T, Motomura A, Hatano N, Seki Y. Fungal internal carotid artery aneurysm treated by trapping and high-flow bypass: A case report and literature review. Neurol Med Chir (Tokyo). 2016. 56: 89-94