- Department of Neurosurgery, Università Politecnica Delle Marche Facoltà di Medicina e Chirurgia, Ancona, Italy
- Department of Maxillo-Facial Surgery, Ospedali Riuniti di Ancona, Ancona, Italy
- Department of Neurosurgery, Università Politecnica delle Marche, Ancona, Italy.
Correspondence Address:
Maria Rossella Fasinella, Department of Neurosurgery, Università Politecnica Delle Marche Facoltà di Medicina e Chirurgia, Ancona, Italy.
DOI:10.25259/SNI_246_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Maurizio Gladi1, Alessandro Di Rienzo1, Maria Rossella Fasinella1, Denis Aiudi1, Paolo Balercia2, Mauro Dobran1, Maurizio Iacoangeli3. Ruptured proximal pontine artery aneurysm and association with cerebellopontine angle cistern arterial venous malformation fed by the same artery: A surgical challenge. 06-Oct-2023;14:352
How to cite this URL: Maurizio Gladi1, Alessandro Di Rienzo1, Maria Rossella Fasinella1, Denis Aiudi1, Paolo Balercia2, Mauro Dobran1, Maurizio Iacoangeli3. Ruptured proximal pontine artery aneurysm and association with cerebellopontine angle cistern arterial venous malformation fed by the same artery: A surgical challenge. 06-Oct-2023;14:352. Available from: https://surgicalneurologyint.com/surgicalint-articles/12586/
Abstract
Background: The coexistence of hyper-inflow aneurysms and cerebellopontine angle cistern (CPAc) arterial venous malformations (AVMs) have been rarely reported and most commonly associated with high risk of bleeding.
Case Descriptions: We present two cases of CPAc AVMs admitted for acute subarachnoid hemorrhage from rupture of a parent right pontine artery aneurysm. Admission history, neurology at presentation, pre/post-operative imaging, approach selection, and results are thoroughly reviewed and presented. The acute origin angle of the vessel from the basilar artery made both malformations unsuitable for endovascular treatment. The surgical strategy was differently tailored in the two patients, respectively, using a Le Fort I/transclival and a Kawase approach. The aneurysm was clipped in the first case, and the AVM was excised in the second one, as required by the anatomical context. Aneurysm exclusion and AVM size reduction were obtained in the first case, while complete AVM removal and later aneurysm disappearance were obtained in the second one. A high-flow cerebrospinal fluid leak in the first case was successfully treated by an endoscopic approach. Both patients experienced a satisfactory neurological outcome in the follow-up.
Conclusion: Pontine artery aneurysms, especially when associated with CPAc AVMs, represent a surgical challenge, due to their rarity and anatomical peculiarity, which typically requires complex operative approaches. Multimodal preoperative imaging, appropriate timing, and accurate target selection, together with versatile strategies, are the keys to a successful treatment.
Keywords: Aneurysm, Arteriovenous malformation, Cerebellopontine angle, Clipping, Pontine artery
INTRODUCTION
Only 8–12% of intracranial aneurysms and 5–15% of arterial venous malformations (AVMs) occur in the posterior circulation.[
The categorization of these aneurysms is based on their relationship with the nidus, extranidal ones more frequently occur on the pedicle of the feeding artery and are considered the leading cause of bleeding in about 37% of cases.[
CASE DESCRIPTIONS
Illustrative case 1
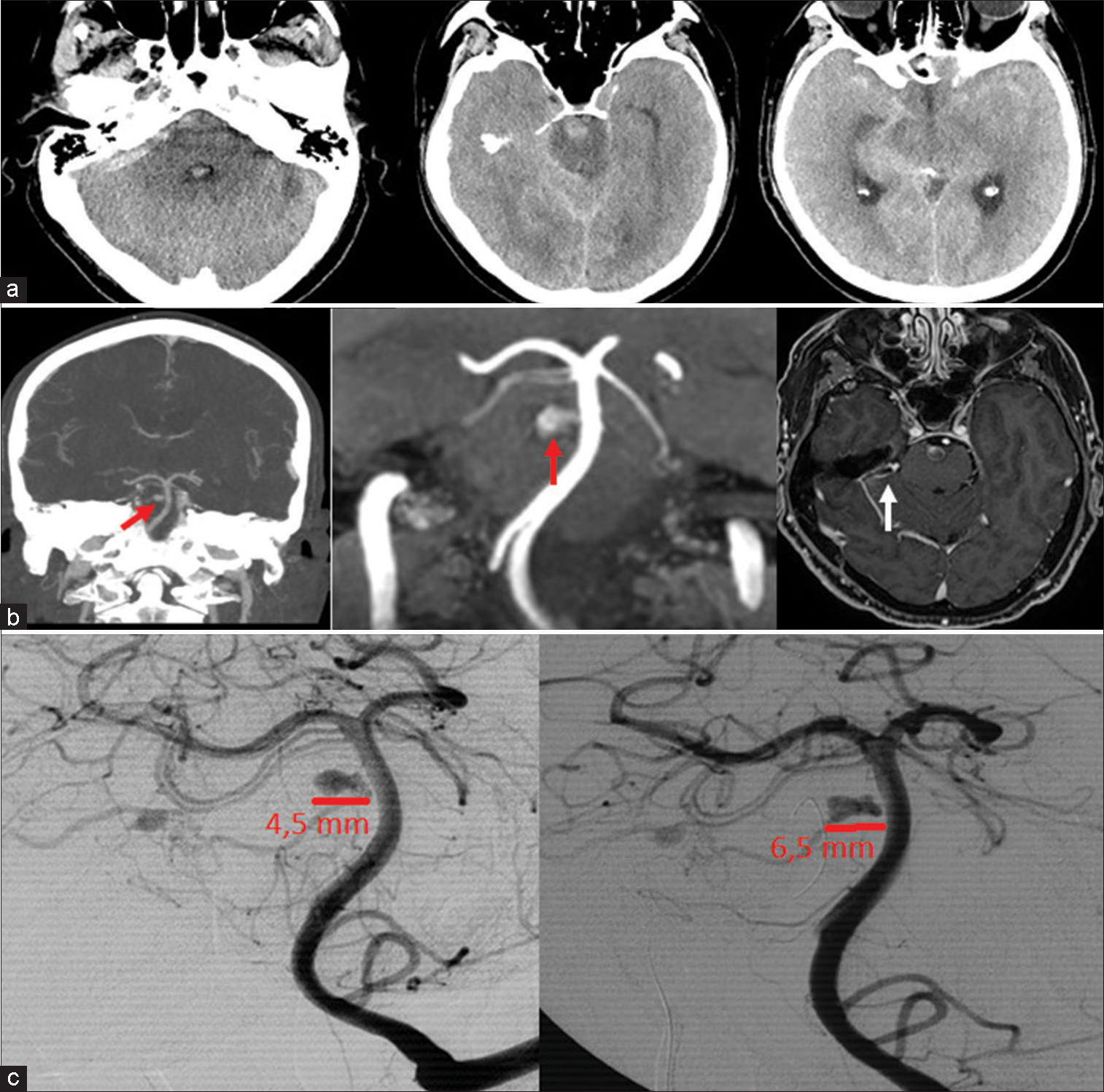
A 63 year old patient was admitted for acute onset of nuchal headache, vomiting, and dysarthria. The emergency head computerized tomography (CT) scan showed the presence of subarachnoid and intraventricular hemorrhage, predominantly located inside the right CPA and prepontine cisterns. The following CT-angiography (CTA), completed with magnetic resonance angiography (MRA) and digital subtraction angiography (DSA), disclosed the presence of a small right-sided CPAc AVM, with the ipsilateral dilation of a parent pontine artery and a hyper-inflow aneurysm (maximum diameter 4.5 mm), deep-seated in the brainstem, at its takeoff from the basilar artery (BA). A second aneurysm was also appreciated distally to the nidus. AVM’s venous drainage was supplied from a right anterolateral pericerebellar vein. The proximal aneurysm was recognized as the leading source of bleeding, and during the following 3 weeks, its size progressively increased to reach a diameter of 6.5 mm at follow-up DSAs [
Figure 1:
(a) Admission computerized tomography (CT) scan showing subarachnoid hemorrhage involving the prepontine and cerebellopontine cisterns; (b) From the left: CT-angiography coronal view showing a right pontine artery aneurysm (red arrow); magnetic resonance angiography confirming the known aneurysm (red arrow) and a cerebellopontine angle cistern arterial venous malformation (white arrow) (c) admission (left) and 3 weeks (right) digital subtraction angiography demonstrating aneurysm growth from 4.5 mm to 6.5 mm.
Surgical technique
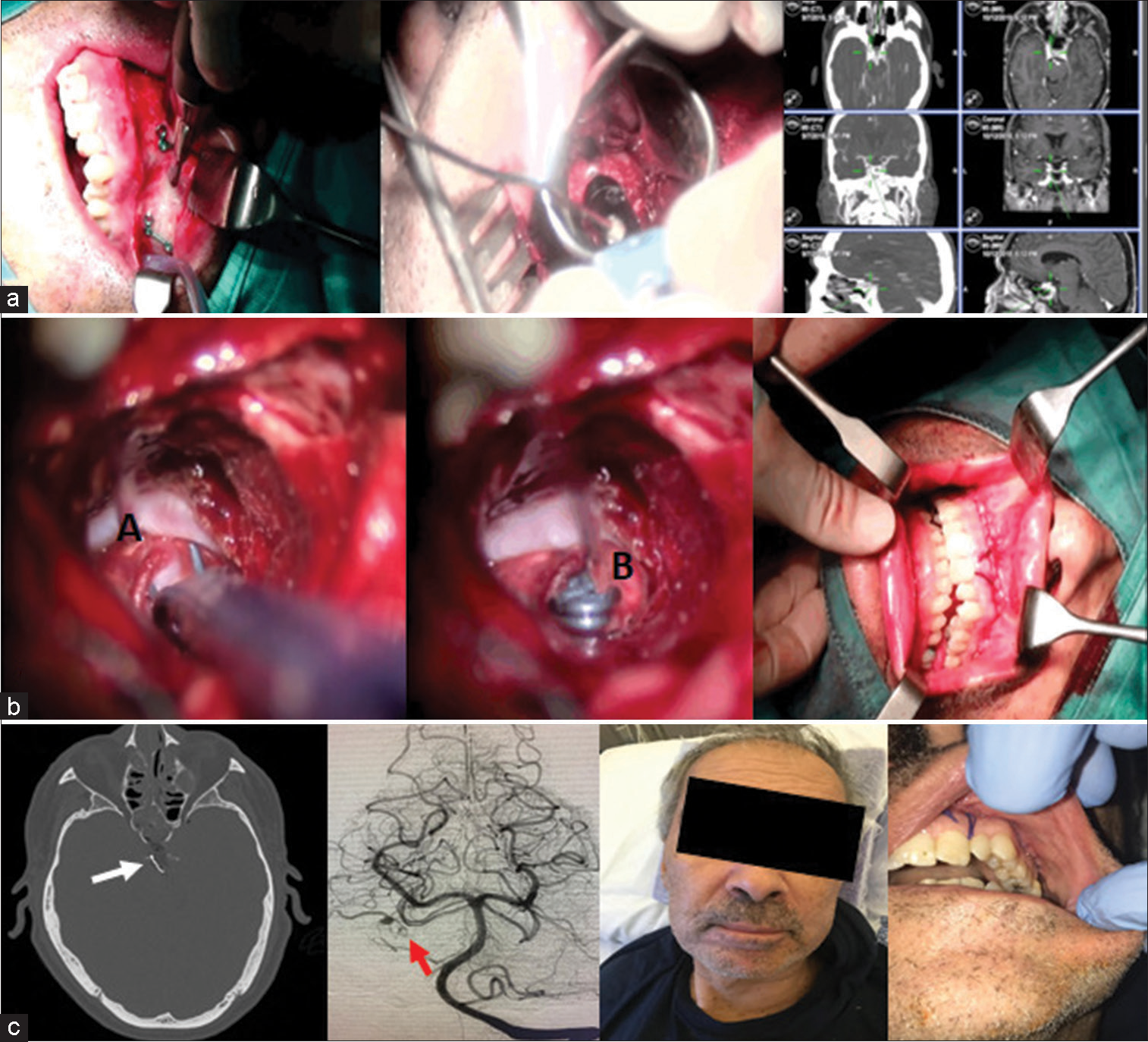
Due to the aneurysm location, a straightforward approach through a median anatomical trajectory was selected. In this context, the transmaxillary Le Fort type I approach was performed, in cooperation with the head and neck surgeons. The oral mucosa and muscles were elevated from the maxilla superiorly, up to the infraorbital nerve. After exposing the piriform opening, the nasal mucosa was stripped, reaching the level of the inferior turbinates. A horizontal osteotomy was made from the piriform opening through the maxillary alveolus using a combination of oscillating saw and piezoelectric osteotome. The maxilla was disarticulated and down-fractured by inserting a chisel and applying downward pressure. The inferior turbinates were fractured and removed with rongeurs. With a tongue retractor holding in place the transected maxilla, the transoral retractor was placed against the palatal plate. The nasal mucosa was reflected off the septum in order to expose the sphenoid base. The clival/paraclival region was finally exposed through a posterior mid-pharyngeal incision. After this step and under navigation guidance, we performed a targeted opening of the sellar floor and clivus, using a combination of drilling and piezosurgery, exposing both the carotids and the posterior clinoid processes. A targeted dural opening led to the identification of the basilar trunk, the parent artery, and the aneurysm. Combining microsurgical and endoscopic techniques, a slightly curved clip was applied, reaching full aneurysm exclusion. The vessel’s patency was verified with a Doppler probe and Indocyanine Green. Adipose tissue was harvested from the abdominal region to close the dural defect and fill the entire sphenoidal sinus. Part of the septum cartilage was used to strengthen the closure, finally reinforced with fibrin glue. The split bone was fixed and re-approximated with titanium mini-plates.
Postoperative DSA confirmed aneurysm exclusion and the parental artery was not appreciable, supposedly due to a stenosis of the vessel itself. Reduced inflow to the distal AVM nidus was still visible, due to retrograde supply from an ipsilateral superior cerebellar artery (SCA) and anterior inferior cerebellar artery (AICA) [
Figure 2:
(a) To the left early mid-facial degloving with piezosurgery, in the middle drilling of the clivus (c), and to the right position check under neuronavigation; (b) aneurysm (A) exposure. The brainstem (B) is visualized beyond the aneurysm dome, final clip position, and (right) closure; (c) computerized tomography bone window demonstrates a tailored bone removal and position of the clip (white arrow). Reduced inflow to the distal AVM nidus is still visible (red arrow). Anterior-posterior angiographic view shows clipping of the aneurysm, which appears completely excluded, as the course of the pontine artery itself due to vessel stenosis. To the right final cosmetic result.
Illustrative case 2
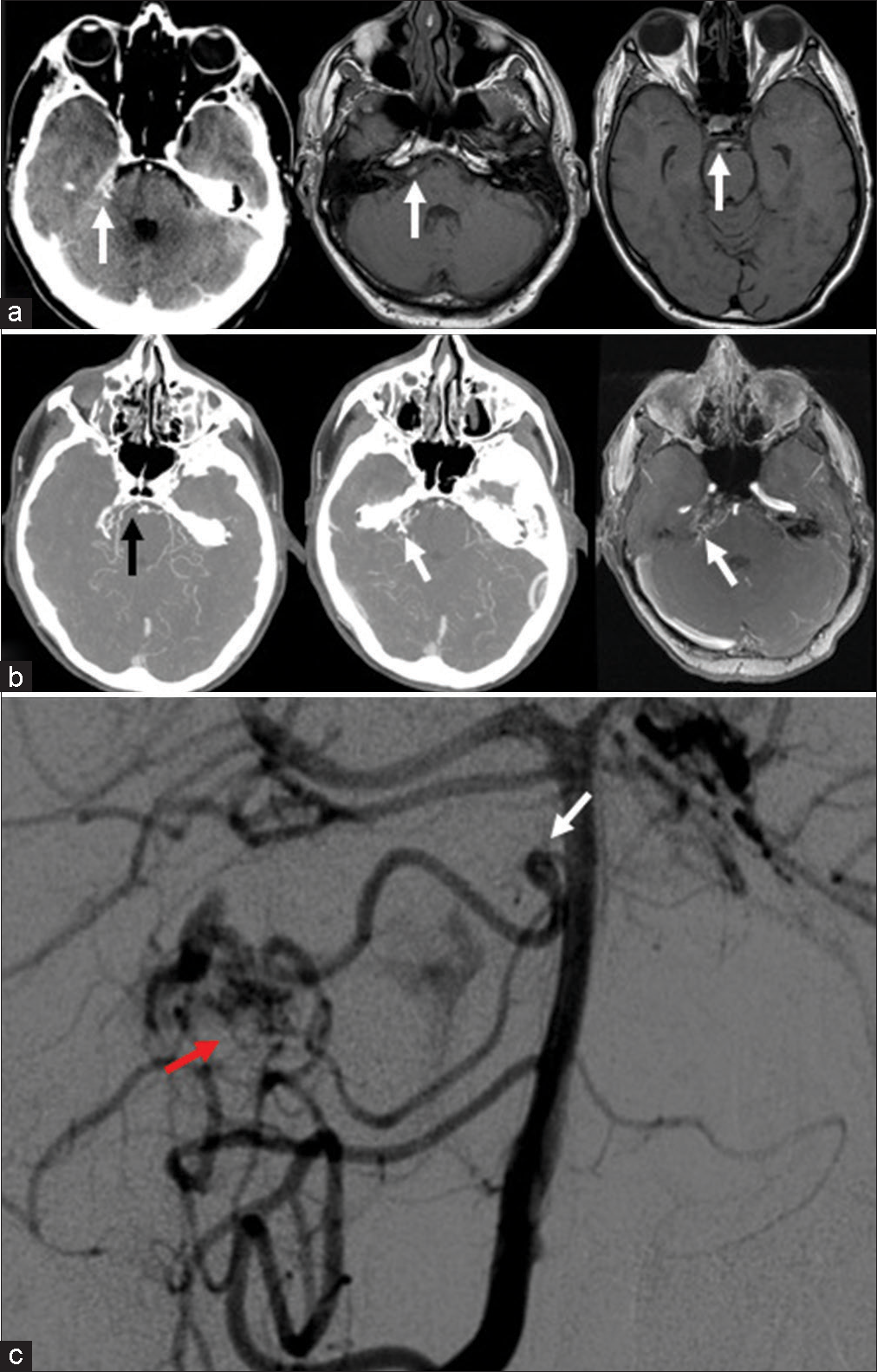
A 48-year-old man admitted to our emergency room with severe nuchal headache and no neurological focal deficits underwent a CT scan showing a right cerebellopontine and prepontine cisterns subarachnoid hemorrhage (SAH). The following CTA and MRA were suggestive of a CPAc AVM. A DSA documented a small-sized right CPAc AVM originating from a fusiform dilation at the origin of the pontine artery, the presumed source of the bleeding
[
Figure 3:
(a) Computerized tomography (CT) and axial magnetic resonance imaging showing basal cisterns subarachnoid hemorrhage (white arrow); (b) from the left: CT-angiography and magnetic resonance angiography (axial view). Aneurysm (black arrow) and arterial venous malformation (AVM) (white arrow); (c) Digital subtraction angiography (anterior-posterior view) outlines the specific characteristics of the vascular malformation; small right cerebellopontine angle cistern AVM (red arrow) with proximal aneurysm (white arrow) whose origin was from a dilated pontine artery. Other minor feeders to the AVM seem to arise from the ipsilateral superior cerebellar artery and anterior inferior cerebellar artery.
Surgical technique
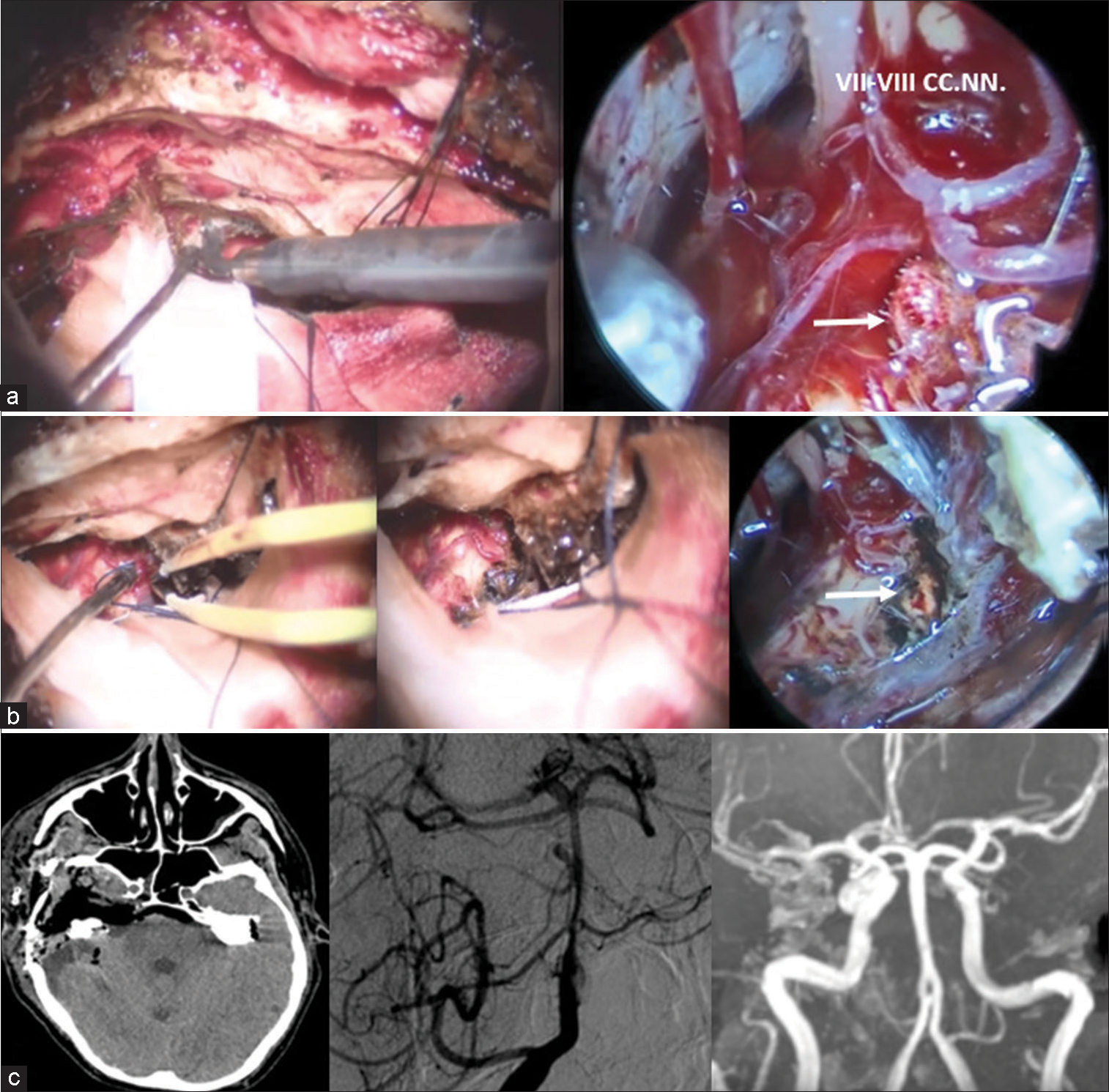
The patient was placed supine with the head turned left. A right subtemporal approach was performed in the standard fashion. The zygomatic arch was exposed, preplated, and removed to pull down the temporalis muscle and gain straight access to the middle cranial fossa. After extradural dissection and CSF subtraction, the bony markers of Kawase’s quadrangle were identified and the petrous apex was drilled. After dural incision and tentorial exposure, the IVth cranial nerve (CN) was identified and the tentorium cut behind it to visualize the posterior cerebral artery, IIIrd CN, and SCA, up to the VIIth–VIIIth CNs. The lateral surface of the brainstem and the AVM was seen. The nidus was isolated and removed after an endoscopic exploration. Finally, a layer-by-layer reconstruction of the middle fossa floor was performed to prevent CSF leakage. The zygomatic arch was finally reapposed.
In the immediate postoperative period, the patient experienced transient diplopia due to a stupor of the VIth right CN, fully resolved 4 weeks later. One week postoperative, DSA confirmed AVM exclusion and downsizing of the aneurysm. Six months later, the aneurysm itself was no longer visible [
Figure 4:
(a) Exposure of the Kawase quadrangle and anterior petrosectomy performed by the use of the piezoelectric osteotome and drilling. On the right, endoscopic close-up view of the arterial venous malformation (AVM) (white arrow); (b) AVM coagulation under microscope and, on the right, final endoscopic assessment of complete AVM exclusion (white arrow); (c) postoperative studies. A baseline computerized tomography scan shows the right anterior petrosectomy. Digital subtraction angiography (anterior-posterior view) performed after 7 days showed complete AVM removal and dimensional reduction of the proximal pontine artery aneurysm. To the right 6-months, magnetic resonance angiography detected no residual vascular anomalies.
DISCUSSION
Posterior fossa AVMs associated with flow-related aneurysms carry a significantly higher rate of rupture and re-bleeding if compared to the isolated aneurysm counterparts.[
Therapeutic intervention for AVMs with associated prenidal aneurysms are complex and multifaceted. As endovascular treatment options have evolved to reduce surgical-related morbidity, the management of posterior circulation aneurysms has significantly changed.[
In our first described case, the endovascular option of a glue embolization was too dangerous due to the absence of a reflux margin, resulting in the risk of distal glue embolization in basilar territory; in the second one, coiling also turned out to be unfeasible in view of the tortuosity of the origin angle of the artery from the basilar trunk, which made the maneuvering space extremely narrow to navigate the pseudoaneurysm and to catheterize the vessel distally to the aneurysm.
GKS is a treatment option for AVMs, but its effect is not immediate and the risk of re-bleeding may persist for up to 2 years.[
To the best of our knowledge, this paper is the first report of two surgically treated patients with proximal pontine artery aneurysms and association with CPAc AVM fed by the same artery. Three more cases were reported in the literature and the onset sign was always SAH. One patient was addressed to GKS, with a residual nidus reduced in size after 16 months. In the remaining two, a combination of aneurysm embolization and AVM radiosurgery was used.[
In our cases, the aneurysms were both placed at the junction between the BA and a dilated pontine artery. This, further, supports the concept that similar lesions might be flow-related rather than linked to an intrinsic vessel wall alteration. A key concept to consider is that both lesions, AVM and aneurysm, are equally exposed to the same high-pressure arterial flow simultaneously since they recognize the same arterial origin.[
The treatment of posterior circulation aneurysms includes limited surgical options, due to the narrow angles of approach and the overall difficulty in ensuring adequate proximal control. Microsurgical clipping of pontine artery aneurysms is especially challenging due to their location within the prepontine cistern, requiring penetration along a deep and narrow surgical corridor,[
Several skull base approaches, including the far-lateral, retrosigmoid, presigmoid (with different degrees of petrosectomy), subtemporal, anterior transpetrosal, orbitozygomatic, and trans-oral/facial/clival,[
Since the choice of the approach is of paramount importance, two factors seem to be strategical to this aim: (1) the craniocaudal location of the aneurysm in relation to the clivus and (2) the mediolateral extension in relation to the course of the artery. The relative position of the VIth CN with respect to the aneurysm may play a role itself in this choice.[
First reported by Sano in 1979, the transclival route to the BA for clipping of cerebral aneurysms was subsequently described by other groups.[
Laterally directed approaches were first popularized by Drake,[
In both patients, the use of endoscopic assistance was crucial to better define locoregional anatomy, highlighting the relationship between the lesion and the vital surrounding structures and to assess the optimal clip placement. Its simultaneous use of microscopic vision was essential to avoid damages related to retraction and unnecessary dissection.[
CONCLUSION
Proximal pontine artery aneurysms and association with CPAc AVMs fed by the same artery are rare and life-threatening lesions. Choosing the type and timing of treatment is strategic, with surgical options being limited due to the complexity of adjacent neurovascular anatomy. Tailoring treatment is necessary and requires a multidisciplinary team, including neuro, head and neck, and ENT surgeons, interventional neuroradiologists, anesthetists, physiotherapists, and radiosurgeons, to optimize the final outcome.
Authors contributions
All authors contributed to the study’s conception and design.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Committee and with the latest amendment of the Helsinki Declaration.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bambakidis NC, Manjila S, Dashti S, Tarr R, Megerian CA. Management of anterior inferior cerebellar artery aneurysms: An illustrative case and review of literature. Neurosurg Focus. 2009. 26: E6
2. Batjer H, Suss RA, Samson D. Intracranial arteriovenous malformations associated with aneurysms. Neurosurgery. 1986. 18: 29-35
3. Case D, Kumpe D, Cava L, Neumann R, White A, Roark C. Ruptured distal posterior inferior cerebellar artery (PICA) aneurysms associated with cerebellar arterial venous malformations (AVMs): A case series and review of the literature demonstrating the need for angiographic evaluation and feasibility of endovascular treatment. World Neurosurg. 2017. 97: 751.e7-13
4. Perneczky A, Fries G. Endoscope-assisted brain surgery: Part 1--evolution, basic concept, and current technique. Neurosurgery. 1998. 42: 219-24 discussion 224-5
5. Crockard HA, Koksel T, Watkin N. Transoral transclival clipping of anterior inferior cerebellar artery aneurysm using new rotating applier. Technical note. J Neurosurg. 1991. 75: 483-5
6. Cronqvist S, Troupp H. Intracranial arteriovenous malformation and arterial aneurysm in the same patient. Acta Neurol Scand. 1966. 42: 307-16
7. Cunha e Sa MJ, Stein BM, Solomon RA, McCormick PC. The treatment of associated intracranial aneurysms and arteriovenous malformations. J Neurosurg. 1992. 77: 853-9
8. Drake CG. The surgical treatment of vertebral-basilar aneurysms. Clin Neurosurg. 1969. 16: 114-69
9. Fischer J, Mustafa H. Endoscopic-guided clipping of cerebral aneurysms. Br J Neurosurg. 1994. 8: 559-65
10. Fries G, Perneczky A. Endoscope-assisted brain surgery: Part 2--analysis of 380 procedures. Neurosurgery. 1998. 42: 226-32 discussion 231-2
11. Gardner PA, Vaz-Guimaraes F, Jankowitz B, Koutourousiou M, Fernandez-Miranda JC, Wang EW. Endoscopic endonasal clipping of intracranial aneurysms: Surgical technique and results. World Neurosurg. 2015. 84: 1380-93
12. Gonzalez LF, Alexander MJ, McDougall CG, Spetzler RF. Anteroinferior cerebellar artery aneurysms: Surgical approaches and outcomes--a review of 34 cases. Neurosurgery. 2004. 55: 1025-35
13. Gonzalez LF, Amin-Hanjani S, Bambakidis NC, Spetzler RF. Skull base approaches to the basilar artery. Neurosurg Focus. 2005. 19: E3
14. He L, Gao J, Thomas AJ, Fusco MR, Ogilvy CS. Disappearance of a ruptured distal flow-related aneurysm after arteriovenous malformation nidal embolization. World Neurosurg. 2015. 84: 6.e1-6
15. Iacoangeli M, Colasanti R, Esposito D, Di Rienzo A, di Somma L, Dobran M. Supraorbital subfrontal trans-laminar endoscope-assisted approach for tumors of the posterior third ventricle. Acta Neurochir (Wien). 2017. 159: 645-54
16. Iacoangeli M, Di Rienzo A, di Somma LG, Moriconi E, Alvaro L, Re M. Improving the endoscopic endonasal transclival approach: The importance of a precise layer by layer reconstruction. Br J Neurosurg. 2014. 28: 241-6
17. Iacoangeli M, Di Rienzo A, Re M, Alvaro L, Nocchi N, Gladi M. Endoscopic endonasal approach for the treatment of a large clival giant cell tumor complicated by an intraoperative internal carotid artery rupture. Cancer Manag Res. 2013. 5: 21-4
18. Iacoangeli M, Nocchi N, Nasi D, Di Rienzo A, Dobran M, Gladi M. Minimally invasive supraorbital key-hole approach for the treatment of anterior cranial fossa meningiomas. Neurol Med Chir (Tokyo). 2016. 56: 180-5
19. Kalavakonda C, Sekhar LN, Ramachandran P, Hechl P. Endoscope-assisted microsurgery for intracranial aneurysms. Neurosurgery. 2002. 51: 1119-26 discussion 1126-7
20. Kano H, Kondziolka D, Flickinger JC, Yang HC, Flannery TJ, Niranjan A. Stereotactic radiosurgery for arteriovenous malformations, Part 5: Management of brainstem arteriovenous malformations. J Neurosurg. 2012. 116: 44-53
21. Khayat HA, Alshareef F, Alshamy A, Algain A, Alhejaili E, Alnabihi O. Pure endovascular management of an arteriovenous malformation and an aneurysm both supplied by anterio-inferior cerebellar artery: A case report and a review of literature. Front Neurol. 2017. 8: 382
22. Kim M, Pyo S, Jeong Y, Lee S, Jung Y, Jeong H. Gamma Knife surgery for intracranial aneurysms associated with arteriovenous malformations. J Neurosurg. 2006. 105: 229-34
23. Koulouris S, Rizzoli HV. Coexisting intracranial aneurysm and arteriovenous malformation: Case report. Neurosurgery. 1981. 8: 219-22
24. Lawton MT, Hamilton MG, Spetzler RF. Multimodality treatment of deep arteriovenous malformations: Thalamus, basal ganglia, and brain stem. Neurosurgery. 1995. 37: 29-36
25. Lee SH, Koh JS, Bang JS, Kim GK. A case of ruptured peripheral aneurysm of the anterior inferior cerebellar artery associated with an arteriovenous malformation: A less invasive image-guided transcortical approach. J Korean Neurosurg Soc. 2009. 46: 577-80
26. Lee SJ, Koh JS, Ryu CW, Lee SH. Ruptured intrameatal aneurysm of the anterior inferior cerebellar artery accompanying an arteriovenous malformation: A case report. Cerebellum. 2012. 11: 808-12
27. Li X, Zhang D, Zhao J. Anterior inferior cerebellar artery aneurysms: Six cases and a review of the literature. Neurosurg Rev. 2012. 35: 111-9
28. Lockwood J, Scullen T, Mathkour M, Kaufmann A, Medel R, Dumont AS. Endovascular management of a ruptured basilar perforator artery aneurysm associated with a pontine arteriovenous malformation: Case report and review of the literature. World Neurosurg. 2018. 116: 159-62
29. Magro E, Chainey J, Chaalala C, Al Jehani H, Fournier JY, Bojanowski MW. Management of ruptured posterior fossa arteriovenous malformations. Clin Neurol Neurosurg. 2015. 128: 78-83
30. Matsumaru Y, Hyodo A, Tsuboi K, Yoshii Y, Nose T, Anno I. Brainstem arteriovenous malformation with a pedicle aneurysm treated by endovascular surgery and proton-beam radiosurgery--case report. Neurol Med Chir (Tokyo). 1996. 36: 716-20
31. Menovsky T, André Grotenhuis J, Bartels RH. Aneurysm of the anterior inferior cerebellar artery (AICA) associated with high-flow lesion: Report of two cases and review of literature. J Clin Neurosci. 2002. 9: 207-11
32. Nishino K, Hasegawa H, Morita K, Fukuda M, Ito Y, Fujii Y. Clinical characteristics of arteriovenous malformations in the cerebellopontine angle cistern. J Neurosurg. 2017. 126: 60-8
33. Nozaki K, Hashimoto N, Kikuta K, Takagi Y, Kikuchi H. Surgical applications to arteriovenous malformations involving the brainstem. Neurosurgery. 2006. 58: ONS-270-8 discussion ONS-278-9
34. Ogilvy CS, Barker FG 2nd, Joseph MP, Cheney ML, Swearingen B, Crowell RM. Transfacial transclival approach for midline posterior circulation aneurysms. Neurosurgery. 1996. 39: 736-41
35. Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: Classification, incidence, and risk of hemorrhage. J Neurosurg. 1998. 89: 539-46
36. Sano K, Pia HW, Langmaid C, Zierski J, editors. Basilar artery aneurysms: Transoral-transclival approach. Cerebral Aneurysms Advances in Diagnosis and Therapy. New York: Springer-Verlag; 1979. p. 326-8
37. Sekhar LN, Heros RC. Origin, growth, and rupture of saccular aneurysms: A review. Neurosurgery. 1981. 8: 248-60
38. Stevenson GC, Stoney RJ, Perkins RK, Adams JE. A transcervical transclival approach to the ventral surface of the brain stem for removal of a clivus chordoma. J Neurosurg. 1966. 24: 544-51
39. Thompson RC, Steinberg GK, Levy RP, Marks MP. The management of patients with arteriovenous malformations and associated intracranial aneurysms. Neurosurgery. 1998. 43: 202-11