- Department of Surgical Neurology, Research Institute for Brain and Blood Vessels-AKITA, Akita, Japan
Correspondence Address:
Jun Tanabe
Department of Surgical Neurology, Research Institute for Brain and Blood Vessels-AKITA, Akita, Japan
DOI:10.4103/sni.sni_410_16
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Jun Tanabe, Tatsuya Ishikawa, Junta Moroi. Safe time duration for temporary middle cerebral artery occlusion in aneurysm surgery based on motor-evoked potential monitoring. 10-May-2017;8:79
How to cite this URL: Jun Tanabe, Tatsuya Ishikawa, Junta Moroi. Safe time duration for temporary middle cerebral artery occlusion in aneurysm surgery based on motor-evoked potential monitoring. 10-May-2017;8:79. Available from: http://surgicalneurologyint.com/surgicalint-articles/safe-time-duration-for-temporary-middle-cerebral-artery-occlusion-in-aneurysm-surgery-based-on-motor%e2%80%91evoked-potential-monitoring/
Abstract
Background:Temporary vessel occlusion of the parent artery is an essential technique for aneurysm surgery. Our aim was to clarify the safe time for temporary occlusion for aneurysm surgery, that is the “safe time duration” (STD), in which brain tissue exposed to ischemia will almost never fall into even the ischemic penumbra during temporary occlusion of the middle cerebral artery (MCA), and even transient postoperative motor impairment will be rare using intraoperative motor-evoked potentials (MEP).
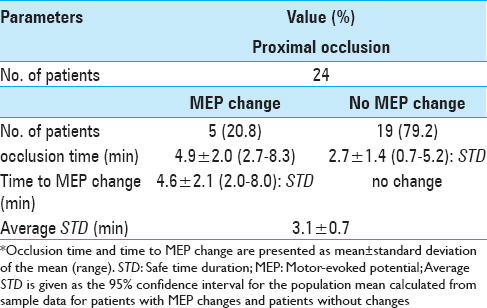
Methods:Twenty-four patients underwent MCA aneurysm clipping surgery with MEP monitoring for 13 ruptured aneurysms and 11 unruptured aneurysms. The duration of vessel occlusion in patients without MEP changes was measured as the STD. Average STD was calculated as 95% confidence interval for the population mean using sample data from patients with MEP changes and patients without changes.
Results:All 24 patients received proximal flow control only. Five patients (20.8%) developed significant intraoperative MEP changes. Time to MEP change (i.e., STD) in these patients was 4.6 ± 2.1 min. In patients without MEP changes, STD was 2.7 ± 1.4 min. Average STD was thus 3.1 ± 0.7 min.
Conclusions:The 95% lower confidence limit for average STD was 2.4 min when applying temporary occlusion on the proximal side of the MCA. This STD resembled that previously reported for temporary proximal occlusion of the internal carotid artery.
Keywords: Aneurysm surgery, middle cerebral artery, motor-evoked potential, temporary vessel occlusion
INTRODUCTION
In aneurysm clipping, temporary vessel occlusion of the parent artery is often necessary to manage aneurysm rupture and facilitate dissection and clip application.[
MATERIALS AND METHODS
Twenty-four patients who had undergone MCA aneurysm clipping surgery with MEP monitoring at our institute were retrospectively included in this study. Thirteen aneurysms were ruptured, and 11 aneurysms were unruptured. Patients with intracranial hemorrhage and/or who had undergone combined bypass surgery were excluded. In the surgeries for ruptured aneurysms, clipping was performed within 72 h after subarachnoid hemorrhage to avoid vasospasm. All 24 patients underwent pterional craniotomy using a transsylvian approach under general anesthesia, and after craniotomy a grid electrode strip with four electrodes (Unique Medical, Tokyo, Japan) was inserted into the subdural space to facilitate electrical stimulation of the motor cortex for the hand. Anesthesia was maintained with continuous injection of propofol at 4–10 mg/kg/h and remifentanil at 0.1–0.3 μg/kg/min. Muscular relaxation was avoided after induction of anesthesia to allow MEP monitoring. We considered that MEP was significantly changed when amplitude decreased to less than 50% when compared with control levels. We performed temporary vessel occlusion to not only avoid uncontrollable aneurysm rupture but also to reduce intra-aneurysm pressure, and thus, facilitate dissection of the aneurysm while avoiding aneurysm rupture. We tried to restore blood flow within no more than 5 min wherever possible. In particular, when the duration of temporary vessel occlusion exceeded 5 min, the next application of temporary occlusion was performed only after maintaining flow to the MCA for more than 5 min. We did not perform any procedures aimed at protecting the brain from ischemia during temporary vessel occlusion. We measured the duration of temporary vessel occlusion in patients without MEP change as the STD. In patients without any MEP changes, the STD could be longer than measured values. In patients who developed MEP changes, the duration until MEP change was recorded as the STD. To estimate average STD, we calculated 95% confidence interval for the population mean from sample data for STD in all patients with MEP changes and in all patients without MEP changes. Average STD represents the estimated value of the virtual population mean based on observed values. For statistical analyses, Student's t-test and Fisher's exact test were used. Differences were considered statistically significant at the P < 0.05 level. Receiver-operating characteristics analysis was used to determine sensitivity and specificity for detecting MEP changes. The abovementioned methods have been described previously.[
RESULTS
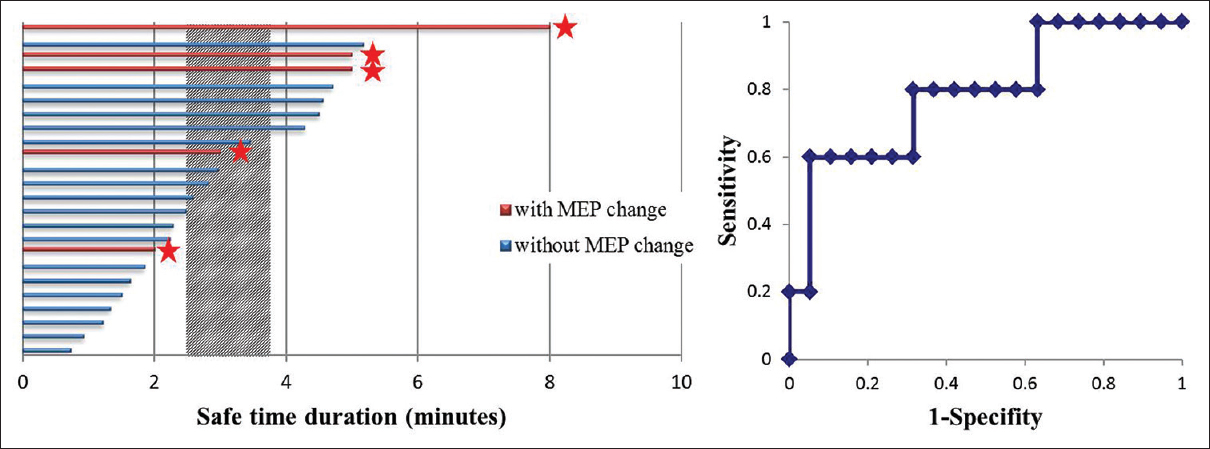
All 24 patients who underwent temporary vessel occlusion of the MCA received proximal flow control only; that is, control at the horizontal segment of the MCA (M1). Five patients (20.8%) developed significant intraoperative MEP changes. In all 5 patients with MEP changes, these changes occurred during the first attempt at vessel occlusion (occlusion time, 4.9 ± 2.0 min). Among these patients, mean time to MEP change (i.e., STD) was 4.6 ± 2.1 min. Occlusion time (i.e., STD) was 2.7 ± 1.4 min in patients without MEP changes [
Figure 1
Histogram of safe time duration (STD) for temporary vessel occlusion. The shaded column demonstrates average STD, representing the 95% confidence interval for the population mean calculated from sample data in patients with motor-evoked potential (MEP) changes and in patients without changes. ★: MEP changes. Receiver-operating characteristic analysis for detecting MEP changes
DISCUSSION
We determined that the 95% lower confidence limit of the average STD was 2.4 min when temporary occlusion was applied at M1. Completing clipping of MCA aneurysms using temporary occlusion within this lower limit of the STD may sometimes prove challenging. However, proximal flow control is not always needed for MCA aneurysms because these aneurysms are not under particularly high pressure and are not adherent to the dura mater in contrast to ICA aneurysms. In many occasions, the temporary clip can be removed within this duration, and then placed again after a couple of minutes as an adequate interval of reperfusion. Consequently, in unruptured cases, each temporary occlusion can be performed within the lower limit of the average STD, even for large aneurysms. In ruptured cases, primary clipping can often be performed during a short temporary occlusion to secure the rupture site, and the final and perfect clipping can be performed in the same manner as for unruptured aneurysms.
Several studies using cortical MEP in a similar manner to the present study have shown that no patients with unchanging MEP experienced postoperative motor paresis during aneurysm surgery.[
We believe the safe time for temporary occlusion in aneurysm surgery (that is, the STD) does not exceed the abovementioned duration of 2.4 min. This is because critical ischemia (in other words, ischemic penumbra) in the MCA territory caused by temporary MCA occlusion results in significant MEP changes, and because the ischemic penumbra can cause transient neurological deficits as a result of some degree of reperfusion injury without developing postoperative brain infarction. In other words, when temporary occlusion is performed within this duration, even transient postoperative motor impairments will rarely occur. Consequently, the STD for temporary vessel occlusion presented here is shorter than previously proposed because previous studies have estimated safe occlusion times based primarily on the presence of postoperative brain infarction, neurological deficit, or SEP changes, which offer lower sensitivity for postoperative motor deficit than MEP changes.[
In this study, the STD of M1 temporary occlusion was slightly shorter than that of ICA temporary occlusion, which has been previously reported (ICA temporary occlusion: time to MEP change, 6.0 ± 2.5 min; average STD, 4.0 ± 0.6 min).[
In this preliminary study, we tried to simply estimate STD and thus did not take into account several factors, as follows. First, when we apply temporary clips to the MCA, ischemic tolerance to proximal occlusion is likely dependent on the existence of leptomeningeal anastomosis as part of the cerebrovascular collateral circulation. Well-developed posterior cerebral arteries, such as those seen with a fetal-type posterior communicating artery, can cause a much more robust collateral circulation. Collateral circulation is difficult to accurately estimate in the clinical setting, particularly before emergent surgery. In addition, although we did not raise blood pressure during temporary occlusion, intentional increases in blood pressure may elevate collateral flow. Second, temporary M1 occlusion proximal to the lenticulostriate artery (LSA) feeding the pyramidal tract, which theoretically results in lower perfusion to the LSA, may shorten the STD. However, we cannot accurately determine where the LSA feeding the pyramidal tract exists on the MCA and cannot intraoperatively evaluate whether the location of temporary occlusion at M1 is proximal or distal to this LSA, particularly in the presence of subarachnoid hemorrhage. Third, temporary vessel occlusion for ruptured aneurysm may carry a significant risk of shortening the STD, because high intracranial pressure after subarachnoid hemorrhage causes a loss of cerebral blood flow even if outside of the vasospasm period. Release of temporary occlusion based on MEP monitoring may thus be ideal in all patients. However, this duration represents important baseline knowledge for neurosurgeons, allowing relatively safe temporary M1 occlusion within this duration without intraoperative MEP monitoring, as this option is not always available in clinical settings around the world. Additional studies are needed to achieve more definitive values.
CONCLUSIONS
Using intraoperative MEP measures, we determined the STD for temporary occlusion of the MCA, in which brain tissue exposed to ischemia, including the pyramidal tract, almost never falls into even the ischemic penumbra. The 95% lower confidence limit for the average STD is 2.4 min for the MCA occluded proximal to the aneurysm.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgments
The authors would like to thank Kimio Yoshioka and Keita Narita for their invaluable supports in the acquisition of intraoperative data.
References
1. Abdulrauf SI, Vuong P, Patel R, Sampath R, Ashour AM, Germany LM. “Awake” clipping of cerebral aneurysms: Report of initial series. J Neurosurg. 2016. p.
2. Guo L, Gelb AW. The use of motor evoked potential monitoring during cerebral aneurysm surgery to predict pure motor deficits due to subcortical ischemia. Clin Neurophysiol. 2011. 122: 648-55
3. Horiuchi K, Suzuki K, Sasaki T, Matsumoto M, Sakuma J, Konno Y. Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J Neurosurg. 2005. 103: 275-83
4. Ishikawa T. What is the role of clipping surgery for ruptured cerebral aneurysms in the endovascular era? A review of recent technical advances and problems to be solved. Neurol Med Chir (Tokyo). 2010. 50: 800-8
5. Lavine SD, Masri LS, Levy ML, Giannotta SL. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: Time limitation and advantage of brain protection. J Neurosurg. 1997. 87: 817-24
6. Mizoi K, Yoshimoto T. Permissible temporary occlusion time in aneurysm surgery as evaluated by evoked potential monitoring. Neurosurgery. 1993. 33: 434-40
7. Neuloh G, Schramm J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg. 2004. 100: 389-99
8. Ogilvy CS, Carter BS, Kaplan S, Rich C, Crowell RM. Temporary vessel occlusion for aneurysm surgery: Risk factors for stroke in patients protected by induced hypothermia and hypertension and intravenous mannitol administration. J Neurosurg. 1996. 84: 785-91
9. Suzuki K, Kodama N, Sasaki T, Matsumoto M, Konno Y, Sakuma J. Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg. 2003. 98: 507-14
10. Symon L, Branston NM, Strong AJ, Hope TD. The concepts of thresholds of ischaemia in relation to brain structure and function. J Clin Pathol Suppl. 1977. 11: 149-54
11. Symon L, Wang A, Costa E, Silva I, Gentili F. Perioperative use of somatosensory evoked responses in aneurysm surgery. J Neurosurg. 1984. 60: 269-75
12. Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V. Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: Intraoperative changes and postoperative outcome. J Neurosurg. 2006. 105: 675-81
13. Tanabe J, Ishikawa T, Moroi J, Suzuki A. Preliminary study on safe thresholds for temporary internal carotid artery occlusion in aneurysm surgery based on motor-evoked potential monitoring. Surg Neurol Int. 2014. 5: 47-
14. Tanaka S, Tashiro T, Gomi A, Takanashi J, Ujiie H. Sensitivity and specificity in transcranial motor-evoked potential monitoring during neurosurgical operations. Surg Neurol Int. 2011. 2: 111-
15. Taylor CL, Selman WR, Kiefer SP, Ratcheson RA. Temporary vessel occlusion during intracranial aneurysm repair. Neurosurgery. 1996. 39: 893-905