- Department of Neurosurgery, Louisiana State University Health Sciences Center New Orleans, New Orleans, Louisiana, United States.
Correspondence Address:
Kierany B. Shelvin, Department of Neurosurgery, Louisiana State University Health Sciences Center New Orleans, New Orleans, Louisiana, United States.
DOI:10.25259/SNI_887_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: John M. Wilson, Kierany B. Shelvin, Sarah E. Lawhon, George A. Crabill, Ellery A. Hayden, Alan J. Velander. Safety and timing of early therapeutic anticoagulation therapy after craniotomy. 02-Feb-2024;15:31
How to cite this URL: John M. Wilson, Kierany B. Shelvin, Sarah E. Lawhon, George A. Crabill, Ellery A. Hayden, Alan J. Velander. Safety and timing of early therapeutic anticoagulation therapy after craniotomy. 02-Feb-2024;15:31. Available from: https://surgicalneurologyint.com/surgicalint-articles/12730/
Abstract
Background: To date, there are few guidelines and studies to guide the timing of initiation of therapeutic anticoagulation (AC) after craniotomy. The goal of this study was to assess the timing, safety, and outcomes of patients following the administration of therapeutic AC after craniotomy.
Methods: A retrospective case–control study was performed evaluating all craniotomy patients from August 2017 to July 2021. Cases were selected if they received therapeutic AC within ten days of craniotomy. Nineteen out of 1013 craniotomy patients met the inclusion criteria. Indications for therapeutic AC were diverse, including deep venous thrombosis, pulmonary embolism, dural venous sinus thrombosis, mechanical heart valve, and left ventricular thrombus.
Results: The mean and median time to therapeutic AC were 5.35 and 5 days, respectively. Three patients developed intracerebral hemorrhage (ICH) that was stable on repeat imaging and did not require any surgical intervention or result in new neurologic deficits. There was no significant association between therapeutic AC and postoperative ICH (P = 0.067).
Conclusion: This study demonstrated that the initiation of therapeutic AC in postoperative craniotomy patients from postoperative days 2 to 10 did not result in any major complications. A prospective study is warranted to clarify the indications and safety of therapeutic AC after craniotomy.
Keywords: Anticoagulation therapy, Craniotomy, Intracranial hemorrhage, Mechanical heart valves, Venous thromboembolism
INTRODUCTION
Therapeutic anticoagulation (AC) is essential to the management of many diseases, in particular venous thromboembolism (VTE). The incidence of postoperative VTE after craniotomy ranges from 3% to 60% in the literature.[
Intracerebral hemorrhage (ICH) at the surgical site in patients who undergo a craniotomy can result in devastating complications, including reoperation, permanent neurological deficit, and death.[
Whereas earlier studies had investigated therapeutic AC for mechanical heart valves or VTE several weeks after craniotomy, few studies have examined the outcomes of early therapeutic AC.[
MATERIALS AND METHODS
Study design
This protocol was approved by the Institutional Review Board (IRB) at Louisiana State University Health Sciences Center-New Orleans (IRB #2071). This is a single-center retrospective review of neurosurgical patients between August 2017 and July 2021. Inclusion criteria were craniotomy or craniectomy and therapeutic AC in <10 days postoperatively. No exclusion criteria were specified.
Study collection and clinical methods
A total of 1013 underwent craniotomy or craniectomy at our institution in the study period. Ninety-six patients were retrospectively identified in Epic SlicerDicer as possible study candidates. Subsequent chart review identified 40 patients started on therapeutic AC post craniotomy during their hospitalization. Of this number, 19 patients were started on therapeutic AC within ten days. These patients were matched by age (±2 years) and gender in a 1:2 ratio to control craniotomy subjects. Therapeutic AC was defined as therapeutic doses of AC with heparin, enoxaparin, fondaparinux, warfarin, apixaban, rivaroxaban, or dabigatran. Demographics and clinical data included age, gender, reason for admission, date of surgery, type of surgery, disposition, and mortality. Specific AC data included prophylactic measures, therapeutic agents, indication, time from surgery to initiation, complications, and interventions.
In our practice, therapeutic AC with heparin was preferred over other agents due to its short half-life and ready reversibility in patients at risk of postoperative ICH. Our heparin nomogram started at 14 units/kg/hour without boluses and targeted anti-Xa assays of 0.4–0.6 units/mL. Other dosing regimens for therapeutic AC were based on manufacturer recommendations. Surveillance imaging was performed when the anti-Xa level reached the target range or a day after the start of other agents. Subsequent imaging was repeated based on new neurological symptoms.
Statistical analysis
Descriptive statistics were calculated on categorical, discrete, and continuous variables. The chi-square test was calculated to detect an association between therapeutic AC and postoperative ICH in the case versus control groups. Statistical analysis was performed in Excel 2023 (Microsoft) and Statistical Package for the Social Sciences Statistics version 29 (IBM).
RESULTS
Nineteen patients started therapeutic AC within ten days after craniotomy, as detailed in
Seven patients received AC preoperatively. Three patients were on warfarin for mechanical heart valves. Two patients were on apixaban; one patient was on apixaban for atrial fibrillation, while the other was on apixaban for an inferior vena cava (IVC) thrombus. One patient was on unfractionated heparin due to a PE that was found in the hospital before surgery. One patient had metastatic cancer and was on enoxaparin for a deep venous thrombosis (DVT). In preparation for craniotomy, the reversal of AC varied by the urgency of the surgery. In two patients, surgery was elective, and therapeutic AC was held for at least five half-lives without bridging AC before surgery. Of the remaining cases, reversal was performed emergently with prothrombin complex concentrate (PCC) and Vitamin K for warfarin, protamine for heparin, and PCC for apixaban.
In the postoperative period, prophylactic AC was specified by each neurosurgeon. All patients received mechanical prophylaxis with sequential compression devices. Of the remainder, most were started on subcutaneous heparin or enoxaparin. This choice was unit-specific and reflected a concern for rapid reversibility with heparin as compared to increased efficacy with enoxaparin. Prophylactic AC was started on postoperative day (POD) 1–2 in 14 (74%) and POD 1–3 in 17 patients (89%). Of the two other patients, one patient started therapeutic heparin on POD 2, whereas the other patient started therapeutic enoxaparin on POD 3 to treat a DVT.
In every case, the need for AC was determined to be urgent to emergent, either due to new VTE, large clot burden (IVC clot, subacute DVT), new left ventricular thrombus, or highly thrombogenic mechanical heart valve. The initial AC agent was heparin in 13 patients (68%), enoxaparin in three patients (16%), apixaban in two patients (11%), and warfarin in one patient (5%). The average time to start AC after craniotomy was 5.35 days. The fastest time to start AC post craniotomy was two days, while the slowest time was ten days. The most common indication for therapeutic AC was VTE in 13 patients (68%).
Figure 1:
Proposed therapeutic AC algorithm after craniotomy *As determined by consultation with cardiology with features to include left atrial appendage thrombus, left atrial spontaneous echocardiographic contrast, and LV ejection fraction <40%. †Based on institutional and departmental interpretation of common heparin nomograms. AC: Anticoagulation, AF: Atrial fibrillation, DVST: Dural venous sinus thrombosis, ICH: Intracerebral hemorrhage, IVC: Inferior vena cava, LV: Left ventricle, MI: Myocardial infarction, PPX: Prophylaxis. VTE: Venous thromboembolism.
In one case, an ICH was detected after a right cerebellar tumor resection on POD 9 following four days of AC. Her new neurologic deficits were headache and lethargy, and the volume of the hemorrhage was 16 mL. Protamine was given to reverse therapeutic enoxaparin, an IVC filter was placed for her right leg DVT, and further, therapeutic AC was withheld despite a small PE. The patient was restarted on prophylactic AC 5 days after ICH and did not develop any further complications from the VTE or ICH.
The second patient developed a left frontal ICH and intraventricular hemorrhage after a bifrontal DC for TBI. The patient had a mechanical heart valve, and therapeutic heparin as a bridge to warfarin was started on POD 2. The ICH was detected due to new lethargy on POD 16 after 14 days of therapeutic AC and stable imaging twice before. The ICH had a volume of 35 mL in the context of known bifrontal contusions. The patient’s anti-Xa was 0.35, and INR was 1.7 at that time. After reversal with protamine and PCC, repeat imaging was stable, and therapeutic AC was restarted one week later without further complication.
The final patient had a left DC for TBI and demonstrated a small right tentorial subdural hematoma on POD 15. The patient was started on therapeutic heparin as a bridge to warfarin for a mechanical heart valve on POD 5. Repeat imaging was stable, and therapeutic AC was not interrupted.
The only complication of therapeutic AC outside of ICH occurred in a patient on therapeutic heparin who developed heparin-induced thrombocytopenia and thrombosis. The patient was switched to argatroban and then transitioned to apixaban without any further complication.
The therapeutic AC cohort was compared to a control cohort of 38 patients who were not anticoagulated in 10 days of craniotomy, as detailed in Supplemental
DISCUSSION
As the most common fatal postoperative complication of neurosurgery is VTE, it is imperative to identify the safe timing of therapeutic AC after surgery.[
Preliminary evidence to inform this decision is mounting. Prophylactic AC is routinely started after craniotomy and has been shown to reduce the incidence of VTE in 1087 patients after craniotomy.[
Our series adds to the evidence that therapeutic AC within ten days post-craniotomy can be safe. The indications for craniotomy in our population included elective and emergent conditions. The indications for therapeutic AC were similarly diverse, reflecting the complexity of contemporary practice. Our institution primarily performed therapeutic AC with a heparin nomogram without boluses and with a conservative anti-Xa goal. This strategy was conservative, designed to prolong the time from heparin initiation to therapeutic AC to avoid periods of supratherapeutic AC and related complications. While therapeutic enoxaparin is acknowledged to have a lower bleeding risk than heparin in many conditions, this has not yet been established in the neurosurgical literature. With its short half-life and easy reversibility, heparin AC may be preferable in patients at high risk for postoperative ICH. Sixteen percent of subjects developed ICH after therapeutic AC in our study. None resulted in reoperation, morbidity, or mortality, and therapeutic AC was either continued or restarted shortly after that. This outcome compares favorably with other recent series. A larger randomized trial is indicated to clarify the timing, protocol, and safety of therapeutic AC against the risk of ICH after craniotomy.
There are several limitations to our study. The control group was matched by age and gender but not the severity of illness. This weakness would have only supported the alternative conclusion that there was a significantly higher rate of postoperative ICH than without therapeutic AC. Although most patients were started on heparin for therapeutic AC first, there was some heterogeneity. This variation reflects the retrospective nature of our research, which included multiple neurosurgeons with different training and experience. The heterogeneity of indications for craniotomy and AC was even greater and certainly limited the generalizability of our findings, particularly given its small sample size from a single institution. Despite this limitation, the study nonetheless reflects the low current level of evidence in the field and adds to preliminary evidence to inform further investigation. In the future, it will be beneficial to collaborate with other institutions to execute a prospective study that standardizes the AC protocol, increases sample size, and controls for bias and confounding variables. The algorithm in
CONCLUSION
This retrospective study demonstrated the safety of starting therapeutic AC in postoperative craniotomy patients. Approximately 16% of patients developed ICH after starting therapeutic AC, but none of these cases required surgery or resulted in a permanent neurological deficit. A future study should aim to develop a specific protocol and time to initiate therapeutic AC on postoperative craniotomy patients safely.
Ethical approval
This protocol was approved by the Institutional Review Board (IRB) at Louisiana State University Health Sciences Center-New Orleans (IRB #2071 September 22nd, 2021)
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SUPPLEMENTAL TABLES
SUPPLEMENTAL FIGURE
Supplemental Figure 1:
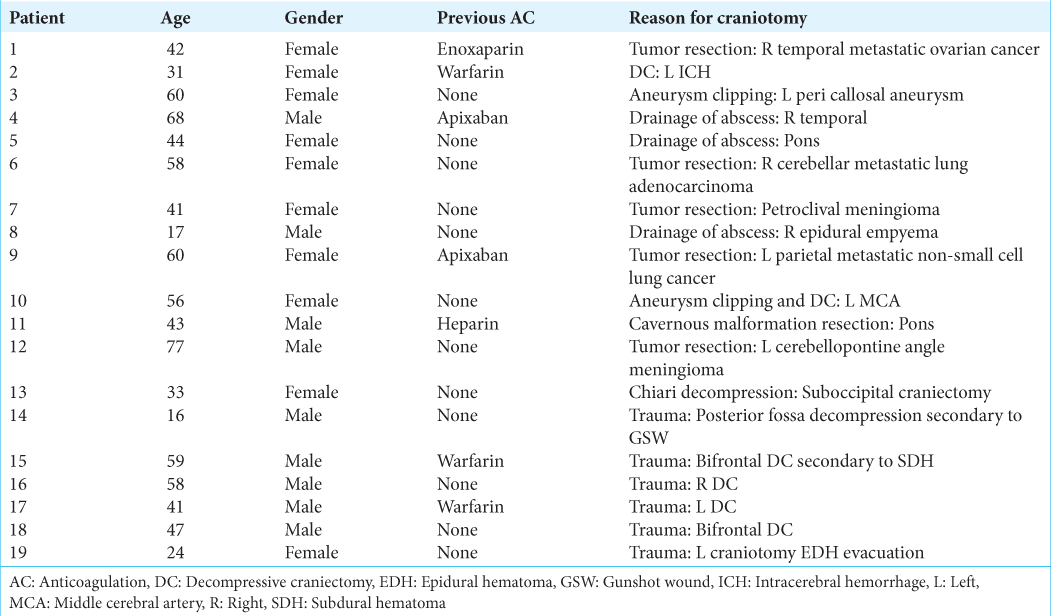
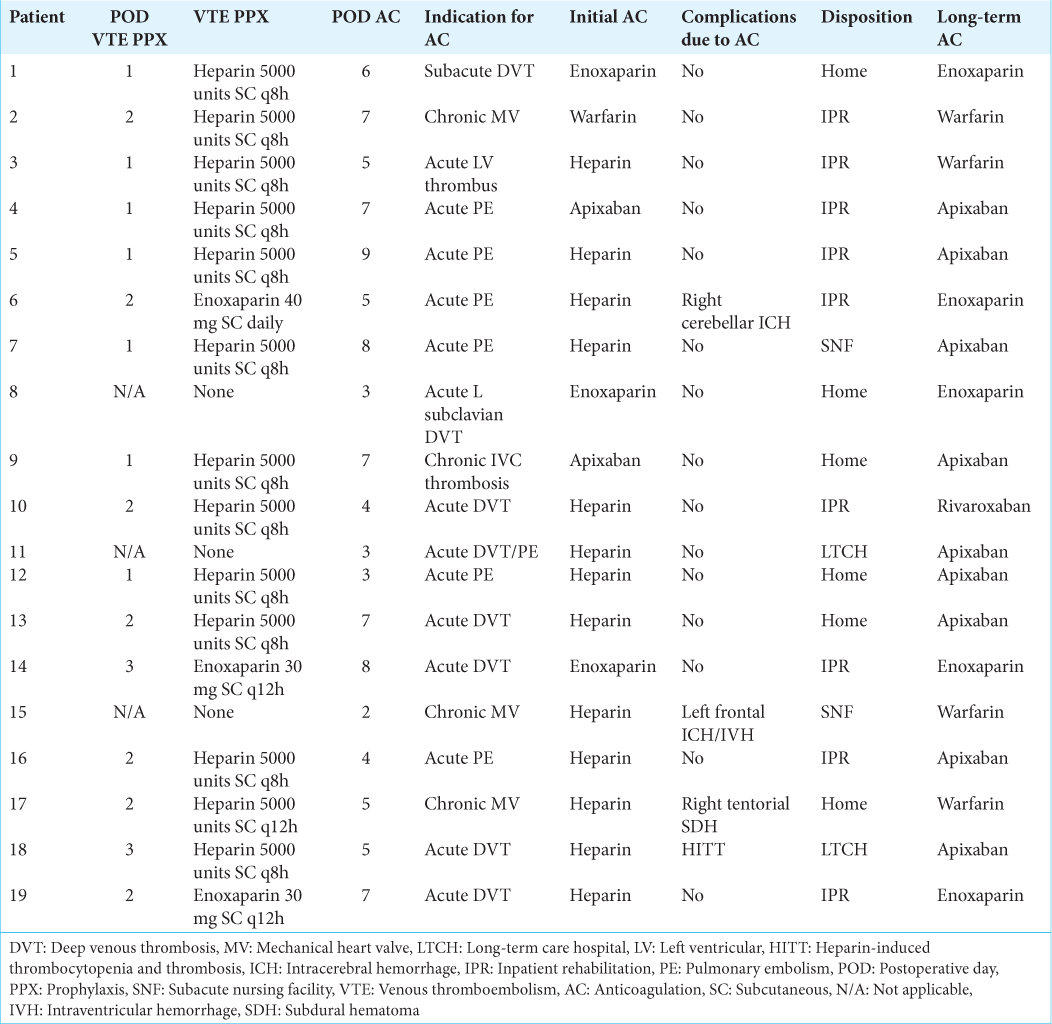
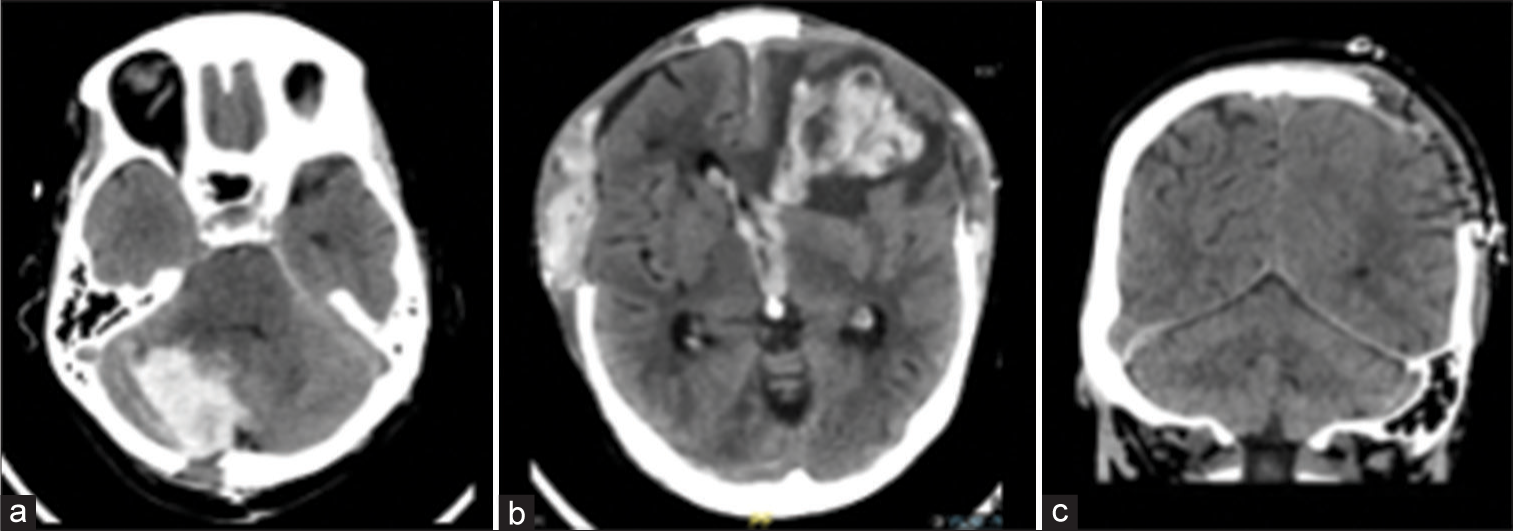
Postoperative intracranial hemorrhage after therapeutic AC Patient 6 in (a) suffered a 16 mL ICH in the resection bed of her right cerebellar metastasis. Patient 15 in (b) developed a 35 mL ICH with intraventricular hemorrhage in the context of bifrontal contusions and bifrontal DC after TBI. Patient 17 in (c) had a minimal right tentorial subdural hematoma after leaving DC for TBI. AC: Anticoagulation, DC: Decompressive craniectomy, ICH: Intracerebral hemorrhage, TBI: Traumatic brain injury.
References
1. Algattas H, Kimmell KT, Vates GE, Jahromi BS. Analysis of venous thromboembolism risk in patients undergoing craniotomy. World Neurosurg. 2015. 84: 1372-9
2. Algattas H, Kimmell KT, Vates GE. Risk of reoperation for hemorrhage in patients after craniotomy. World Neurosurg. 2016. 87: 531-9
3. Amin AG, Ng J, Hsu W, Pradilla G, Raza S, QuinonesHinojosa A. Postoperative anticoagulation in patients with mechanical heart valves following surgical treatment of subdural hematomas. Neurocrit Care. 2013. 19: 90-4
4. Briggs RG, Lin YH, Dadario NB, Young IM, Conner AK, Xu W. Optimal timing of post-operative enoxaparin after neurosurgery: A single institution experience. Clin Neurol Neurosurg. 2021. 207: 106792
5. Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: Case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010. 152: 578-89
6. de Melo Junior JO, Lodi Campos Melo MA, da Silva Lavradas LA, Ferreira Lopes PG, Luiz Ornelas II, de Barros PL. Therapeutic anticoagulation for venous thromboembolism after recent brain surgery: Evaluating the risk of intracranial hemorrhage. Clin Neurol Neurosurg. 2020. 197: 106202
7. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: A report from the Swedish atrial fibrillation cohort study. Circulation. 2012. 125: 2298-307
8. Ganau M, Prisco L, Cebula H, Todeschi J, Abid H, Ligarotti G. Risk of Deep vein thrombosis in neurosurgery: State of the art on prophylaxis protocols and best clinical practices. J Clin Neurosci. 2017. 45: 60-6
9. Hacker E, Ozpinar A, Fernandes D, Agarwal N, Gross BA, Alan N. The utility of routine head CT for hemorrhage surveillance in post-craniotomy patients undergoing anticoagulation for venous thromboembolism. J Clin Neurosci. 2021. 85: 78-83
10. Kaewborisutsakul A, Tunthanathip T, Yuwakosol P, Inkate S, Pattharachayakul S. Incidence and risk factors for venous thromboembolism following craniotomy for intracranial tumors: A cohort study. Asian J Neurosurg. 2020. 15: 31-8
11. Mehta VA, Wang TY, Sankey EW, Howell EP, Goodwin CR, Levy JH. Restarting therapeutic anticoagulation after elective craniotomy for patients with chronic atrial fibrillation: A review of the literature. World Neurosurg. 2020. 137: 130-6
12. Muhlestein WE, Akagi DS, Chotai S, Chambless LB. The impact of presurgical comorbidities on discharge disposition and length of hospitalization following craniotomy for brain tumor. Surg Neurol Int. 2017. 8: 220
13. O’Donnell M, Weitz JI. Thromboprophylaxis in surgical patients. Can J Surg. 2003. 46: 129-35
14. Riviere-Cazaux C, Naylor RM, Van Gompel JJ. Ultra-early therapeutic anticoagulation after craniotomy-A single institution experience. J Clin Neurosci. 2022. 100: 46-51
15. Saadeh Y, Gohil K, Bill C, Smith C, Morrison C, Mosher B. Chemical venous thromboembolic prophylaxis is safe and effective for patients with traumatic brain injury when started 24 hours after the absence of hemorrhage progression on head CT. J Trauma Acute Care Surg. 2012. 73: 426-30
16. Scheller C, Rachinger J, Strauss C, Alfieri A, Prell J, Koman G. Therapeutic anticoagulation after craniotomies: Is the risk for secondary hemorrhage overestimated?. J Neurol Surg A Cent Eur Neurosurg. 2013. 75: 2-6
17. Senders JT, Snijders TJ, van Essen M, van Bentum GM, Seute T, de Vos FY. Length of thromboprophylaxis in patients operated on for a high-grade glioma: A retrospective study. World Neurosurg. 2018. 115: e723-30