- Department of Neurosurgery, Kagoshima University, Kagoshima, Japan,
- Department of Neurosurgery, Diponegoro University, Tembalang, Semarang, Indonesia,
- Pituitary Disorders Center, Kagoshima University Hospital, Kagoshima, Japan
- Department of Diabetes and Endocrine Medicine, Kagoshima University, Kagoshima, Japan
- Department of Obstetrics and Gynecology, Kagoshima University, Kagoshima, Japan
- Department of Hematology and Rheumatology, Kagoshima University, Kagoshima, Japan
- Department of Pathology, Kagoshima University, Kagoshima, Japan
- Department of Diabetes and Endocrinology, Nara Medical University, Kashihara, Nara, Japan.
Correspondence Address:
Shingo Fujio, Department of Neurosurgery, Kagoshima University, Kagoshima, Japan.
DOI:10.25259/SNI_947_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Eri Inoue1, Irfan Kesumayadi1,2, Shingo Fujio1,3, Ryutaro Makino1,3, Tomoko Hanada1,3, Keisuke Masuda1, Nayuta Higa1, Shigeru Kawade3,4, Yuichiro Niihara3,5, Hirosuke Takagi6, Ikumi Kitazono7, Yutaka Takahashi8, Ryosuke Hanaya1. Secondary hypophysitis associated with Rathke’s cleft cyst resembling a pituitary abscess. 01-Mar-2024;15:69
How to cite this URL: Eri Inoue1, Irfan Kesumayadi1,2, Shingo Fujio1,3, Ryutaro Makino1,3, Tomoko Hanada1,3, Keisuke Masuda1, Nayuta Higa1, Shigeru Kawade3,4, Yuichiro Niihara3,5, Hirosuke Takagi6, Ikumi Kitazono7, Yutaka Takahashi8, Ryosuke Hanaya1. Secondary hypophysitis associated with Rathke’s cleft cyst resembling a pituitary abscess. 01-Mar-2024;15:69. Available from: https://surgicalneurologyint.com/surgicalint-articles/12775/
Abstract
Background: Although rare, cases of hypophysitis resembling a pituitary abscess (PA) have been reported. Differential diagnosis between hypophysitis and PA is crucial as the two diseases require different treatments.
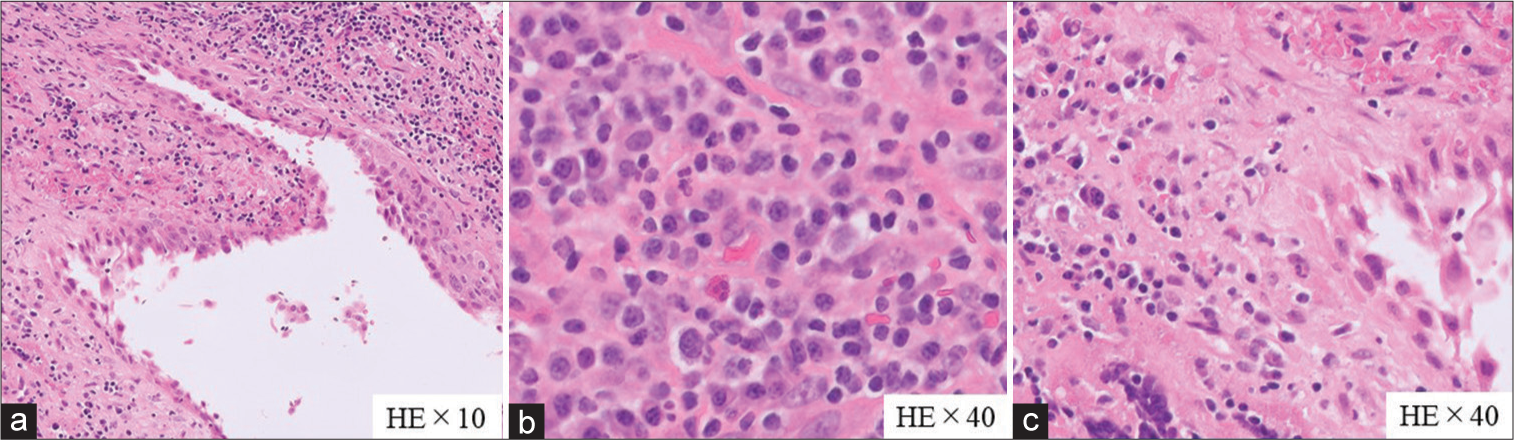
Case Description: A 38-year-old woman with headaches underwent head magnetic resonance imaging (MRI), which revealed an 11-mm mass lesion in the sella turcica. Due to breastfeeding, contrast-enhanced MRI was avoided. Pituitary adenomas and Rathke’s cleft cyst (RCC) were suspected, and she was initially treated conservatively. Five months later, she acquired syndrome coronavirus two infections, and while the fever subsided with acetaminophen, the headache persisted. One month later, the headache worsened, followed by fever and diabetes insipidus. MRI revealed a pituitary cystic mass with ring-shaped contrast enhancement on T1-weighted MRI and increased signal intensity on diffusion-weighted imaging (DWI). PA was suspected, and emergency endoscopic transsphenoidal surgery was performed. The microbiological examination of the yellowish-brown content drained from the cystic mass was negative. Microscopically, the cystic lesion was covered with ciliated columnar epithelium and stratified squamous epithelium, with a dense inflammatory cell infiltrate consisting mainly of lymphocytes and plasma cells observed around the cyst. This supported the diagnosis of secondary hypophysitis associated with RCC without PA.
Conclusion: We report a case of hypophysitis secondary to RCC resembling PA with ring-shaped contrast enhancement on MRI and increased signal intensity on DWI. This case emphasizes the need for cautious diagnosis of secondary hypophysitis due to RCC in individuals with MRIs and clinical manifestations resembling an abscess.
Keywords: Coronavirus disease 2019, Hypophysitis, Pituitary abscess, Rathke’s cleft cyst
INTRODUCTION
Hypophysitis is characterized by pituitary dysfunction resulting from acute or chronic inflammatory conditions.[
Diagnosing hypophysitis is challenging due to its rarity, affecting only approximately 1 in 9 million individuals per year [
CASE DESCRIPTION
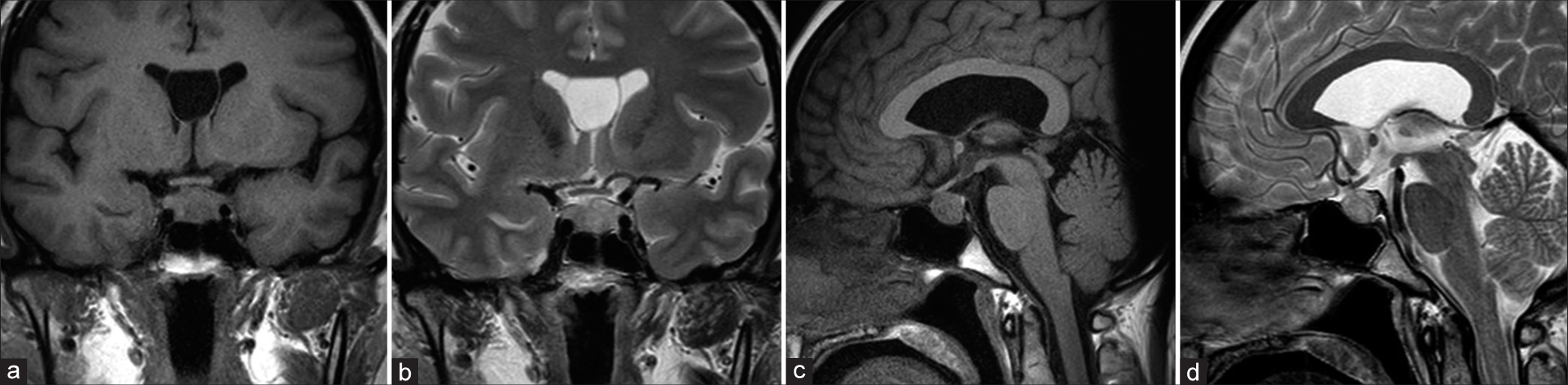
A 38-year-old female patient, who usually suffers from mild headaches, presented with the chief complaint of a sudden severe headache. She was referred to our neurosurgery department after an 11-mm mass lesion was identified in the sella turcica. The patient had given birth one year earlier and was breastfeeding her child. Contrast-enhanced magnetic resonance imaging (MRI) was, therefore, not performed to avoid side effects on infants, as recommended in Japan. Pituitary adenomas and RCC were suspected [
Five months later, after her initial visit to our department, she was infected with SARS-CoV-2, even after receiving the first and second doses of the SARS-CoV-2 vaccine 7 and 6 months earlier, respectively. She had a fever of 38°C and a severe headache that persisted for several days, for which acetaminophen was prescribed. The fever subsided, but the mild headache persisted. One month later, the patient revisited our department, complaining of a headache that had persisted for five days. Head MRI revealed mild enlargement of the lesion and sphenoid sinus mucosal thickening, leading to the diagnosis of pituitary apoplexy [
Figure 2:
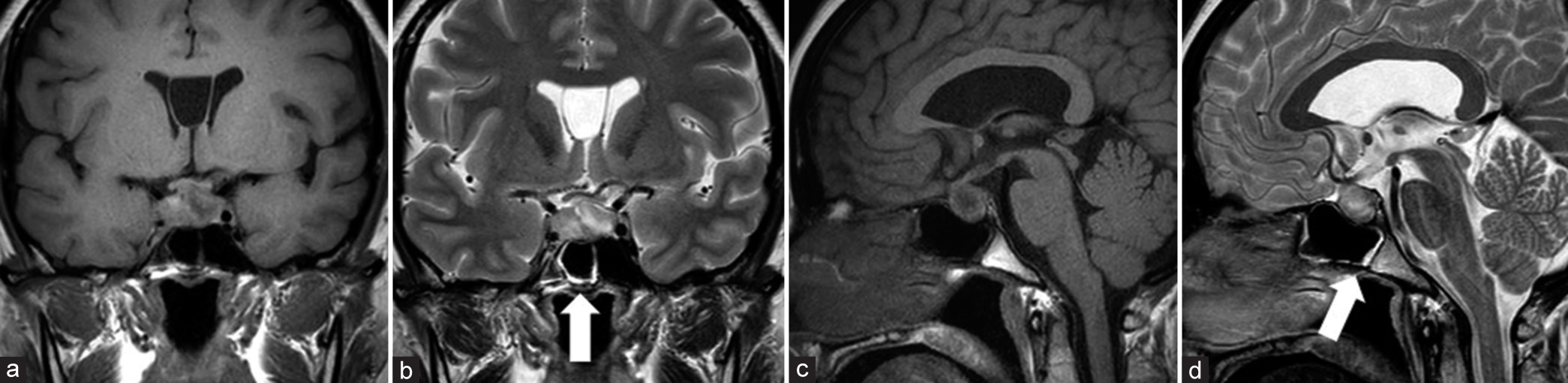
Magnetic resonance imaging findings when the headache worsened showed growth in tumor size. (a) Coronal T1- and (b) T2-weighted images, along with (c) sagittal T1- and (d) T2-weighted images showed a mixture of hypointense and hyperintense signals. Mucosal thickening was observed in the sphenoid sinus (arrows in b and d).
Figure 3:
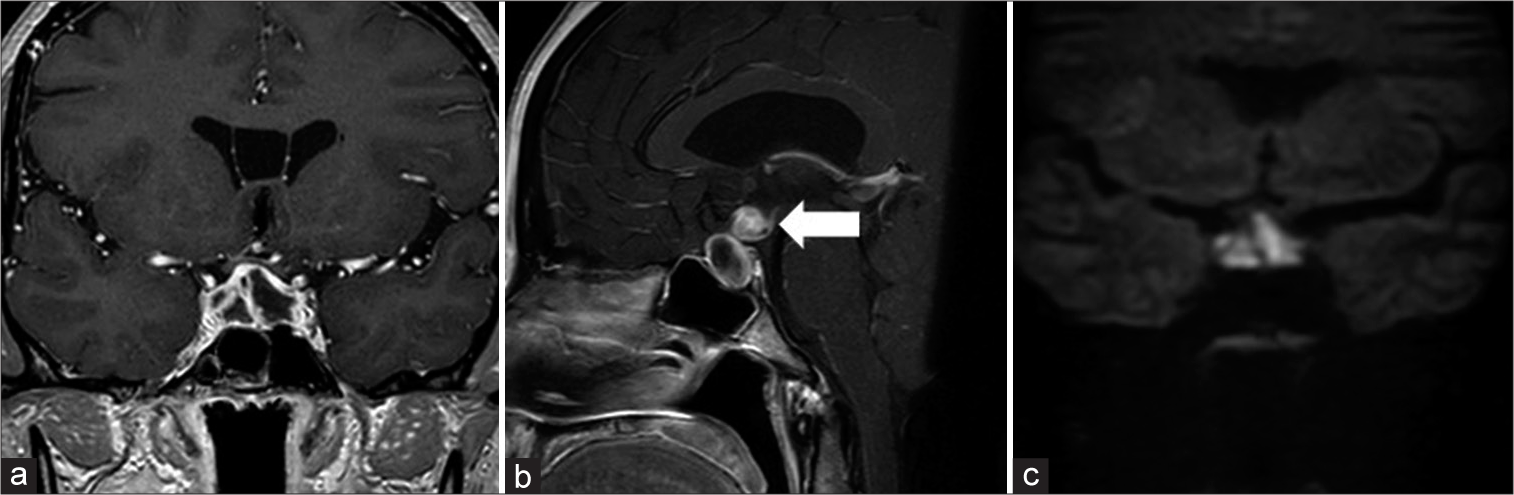
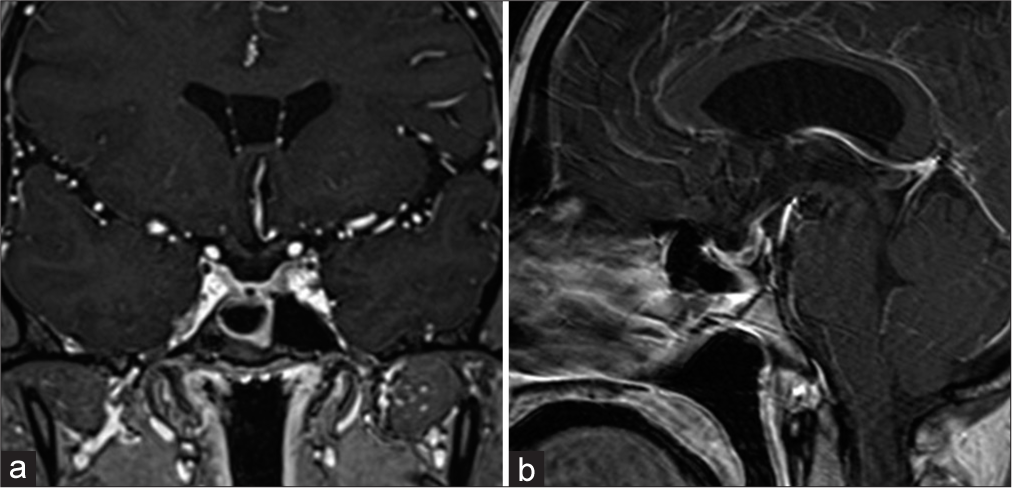
Magnetic resonance imaging findings at the time of fever: (a) coronal; (b) sagittal contrast-enhanced T1-weighted images revealed a ring-shaped contrast enhancement that partially extended into the third ventricle (arrow in b); and (c) coronal diffusion-weighted imaging showed a high signal intensity.
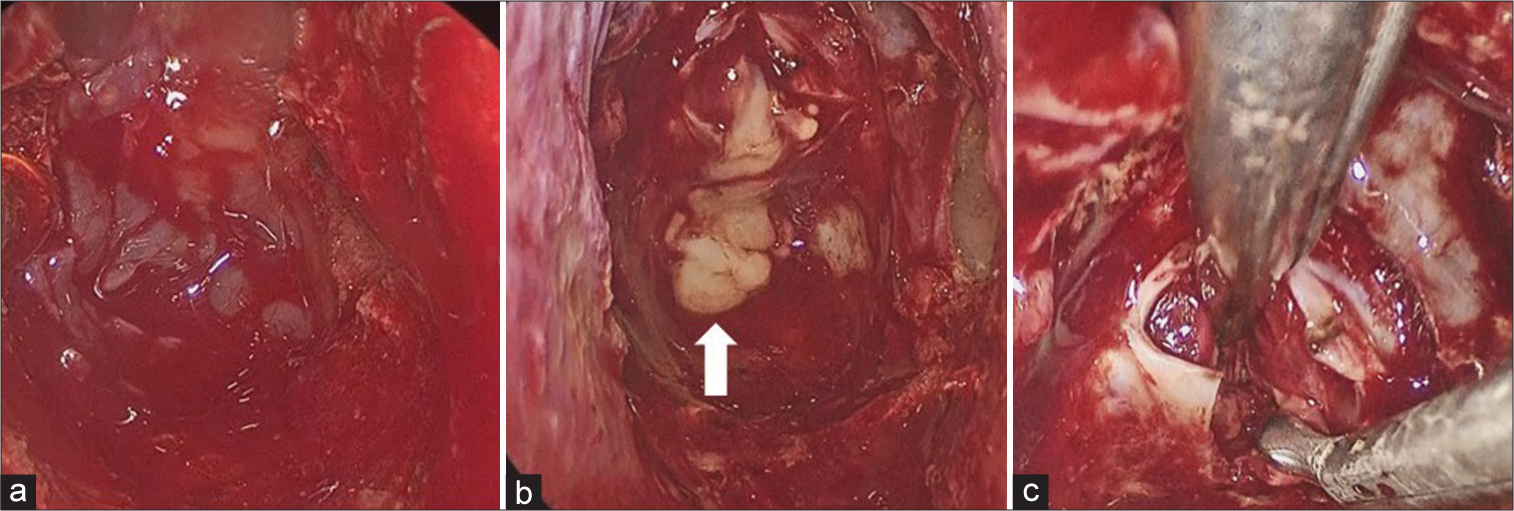
Intraoperative findings showed that the sphenoid sinus mucosa was thickened [
The headache and fever quickly subsided after surgery. The patient was prescribed prednisolone (45 mg/day) immediately after the diagnosis, and the lesions shrank promptly [
DISCUSSION
Our report describes a case of hypophysitis secondary to RCC, with clinical manifestations and MRI findings resembling PA. Along with other pituitary diseases, the incidence of PA is <1% and is associated with a high mortality rate.[
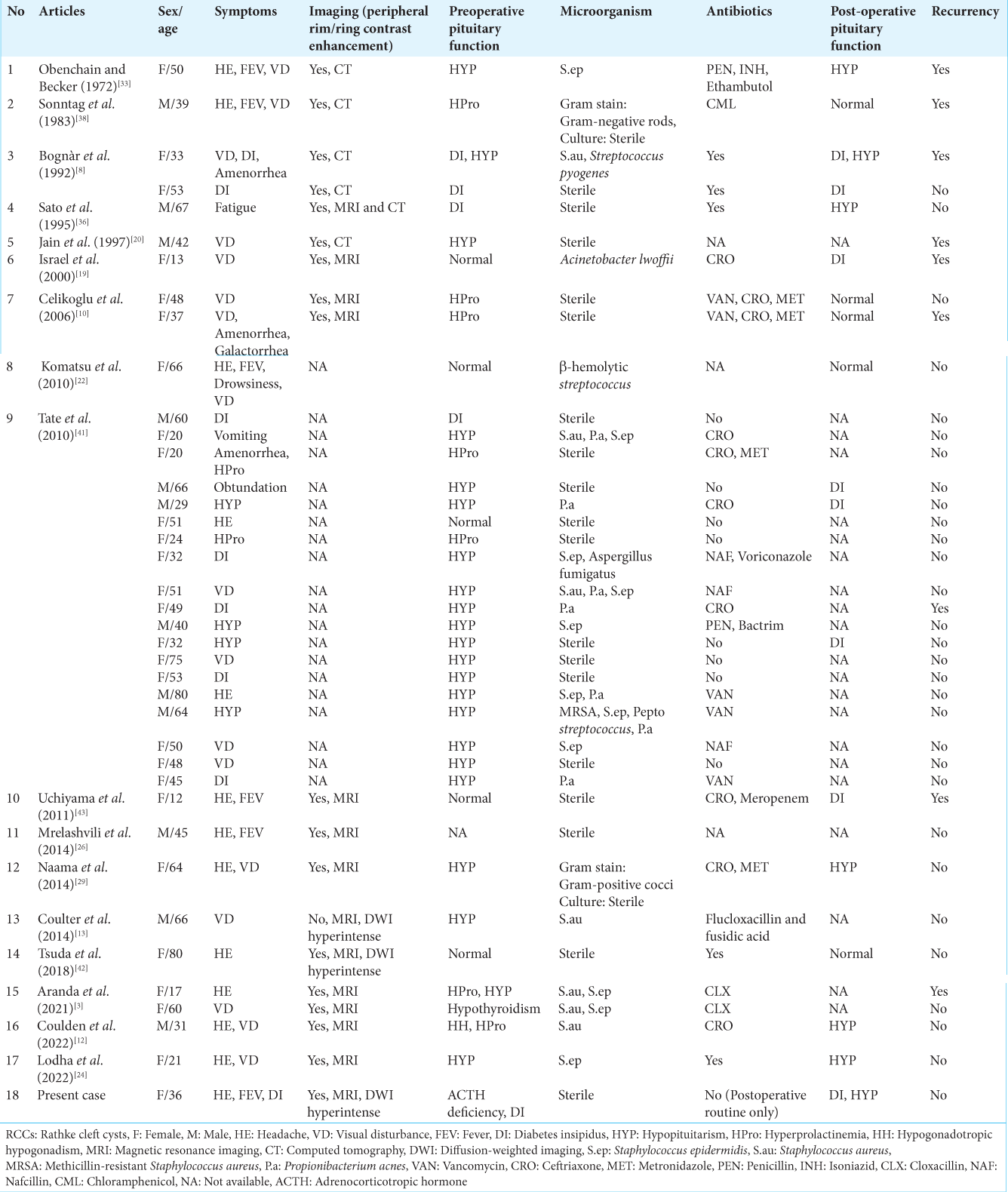
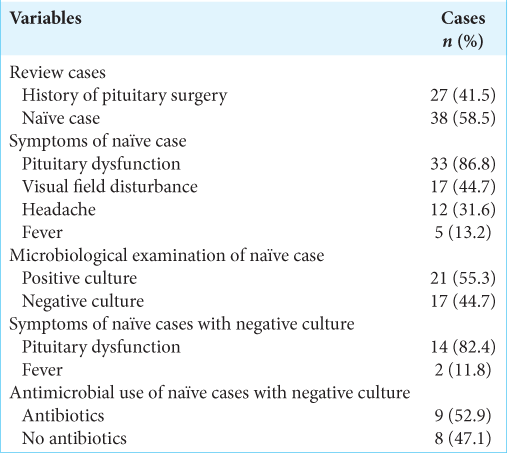
We reviewed cases of RCC reported to be suspected of PA [
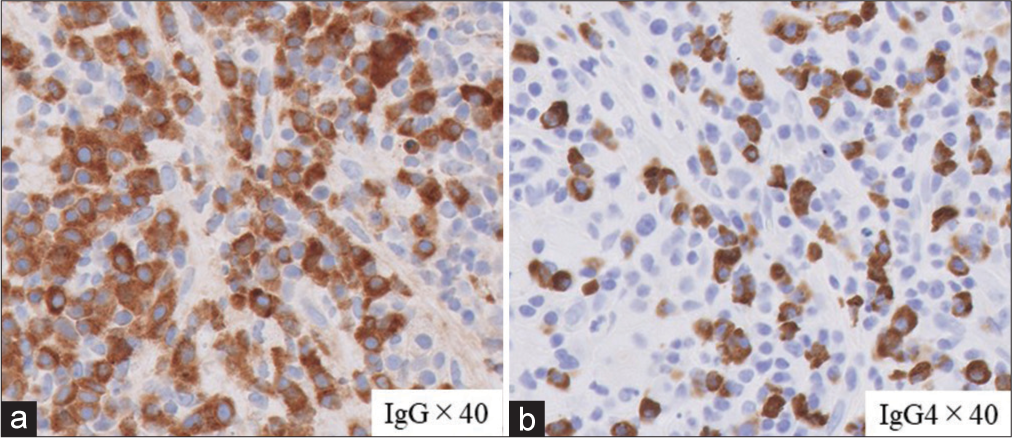
Contrast-enhanced MRI was not performed during the initial examination, considering the patient was breastfeeding. If the contrast MRI had been performed and rim contrast enhancement had been discovered earlier, it would have been useful for aiding the diagnosis. However, given that the headache symptoms improve spontaneously, the therapy administered will remain conservative, with follow-up conducted accordingly. In this case, pathology revealed infiltration of lymphocytes and plasma cells into the anterior pituitary gland, with immunostaining showing massive IgG4-positive cells, mimicking IgG4-RH. In accordance with our case, the previous studies have shown that clusters of IgG4-positive plasmacytes may sometimes be observed in secondary hypophysitis and granulomatosis,[
It is unclear what caused hypophysitis in this patient. It might be related to a SARS-CoV-2 infection encountered a month before symptom exacerbation since hypophysitis as a sequela of coronavirus disease 2019 has been reported in five cases.[
CONCLUSION
We report a case of hypophysitis secondary to RCC with clinical and imaging findings similar to PA. The patient presented with severe headache, fever, and diabetes insipidus. The MRI showed ring-shaped contrast enhancement accompanied by increased signal intensity on DWI. Microbiological examination, however, was negative; pathological findings showed a cystic lesion covered with ciliated columnar epithelium and stratified squamous epithelium. Together, these findings support the diagnosis of hypophysitis secondary to RCC. This case highlights that the diagnosis of hypophysitis secondary to RCC and PA should be made with caution, as both require conflicting treatments.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. AbdelRazek MA, Venna N, Stone JH. IgG4-related disease of the central and peripheral nervous systems. Lancet Neurol. 2018. 17: 183-92
2. Ankireddypalli AR, Chow LS, Radulescu A, Kawakami Y, Araki T. A case of hypophysitis associated with sARS-CoV-2 vaccination. AACE Clin Case Rep. 2022. 8: 204-9
3. Aranda F, García R, Guarda FJ, Nilo F, Cruz JP, Callejas C. Rathke’s cleft cyst infections and pituitary abscesses: Case series and review of the literature. Pituitary. 2021. 24: 374-83
4. Arzt E, Pereda MP, Castro CP, Pagotto U, Renner U, Stalla GK. Pathophysiological role of the cytokine network in the anterior pituitary gland. Front Neuroendocrinol. 1999. 20: 71-95
5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020. 11: 995-8
6. Bando H, Iguchi G, Fukuoka H, Taniguchi M, Kawano S, Saitoh M. A diagnostic pitfall in IgG4-related hypophysitis: Infiltration of IgG4-positive cells in the pituitary of granulomatosis with polyangiitis. Pituitary. 2015. 18: 722-30
7. Bando H, Iguchi G, Fukuoka H, Taniguchi M, Yamamoto M, Matsumoto R. The prevalence of IgG4-related hypophysitis in 170 consecutive patients with hypopituitarism and/or central diabetes insipidus and review of the literature. Eur J Endocrinol. 2013. 170: 161-72
8. Bognàr L, Szeifert GT, Fedorcsàk I, Pàsztor E. Abscess formation in Rathke’s cleft cyst. Acta Neurochir (Wien). 1992. 117: 70-2
9. Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005. 26: 599-614
10. Celikoglu E, Boran BO, Bozbuga M. Abscess formation in Rathke’s cleft cyst. Neurol India. 2006. 54: 213-4
11. Ciappetta P, Calace A, D’Urso PI, De Candia N. Endoscopic treatment of pituitary abscess: Two case reports and literature review. Neurosurg Rev. 2008. 31: 237-46
12. Coulden A, Pepper J, Juszczak A, Batra R, Chavda S, Senthil L. Rathke’s cleft cyst abscess with a very unusual course. Asian J Neurosurg. 2022. 17: 527-31
13. Coulter IC, Mahmood S, Scoones D, Bradey N, Kane PJ. Abscess formation within a Rathke’s cleft cyst. J Surg Case Rep. 2014. 2014: rju105
14. Daikokuya H, Inoue Y, Nemoto Y, Tashiro T, Shakudo M, Ohata K. Rathke’s cleft cyst associated with hypophysitis: MRI. Neuroradiology. 2000. 42: 532-4
15. Dalan R, Leow MK. Pituitary abscess: Our experience with a case and a review of the literature. Pituitary. 2008. 11: 299-306
16. Deguchi-Horiuchi H, Koide H, Sakuma I, Gao Y, Higuchi S, Nagano H. Two cases of symptomatic secondary hypophysitis due to Rathke’s cleft cysts treated with glucocorticoids: Long-term follow-up. Endocr J. 2021. 68: 269-79
17. Faje A. Hypophysitis: Evaluation and management. Clin Diabetes Endocrinol. 2016. 2: 15
18. Gorbova NY, Vladimirova VP, Rozhinskaya LY, Belaya ZY. Hypophysitis and reversible hypopituitarism developed after COVID-19 infection-a clinical case report. Probl Endokrinol (Mosk). 2022. 68: 50-6
19. Israel ZH, Yacoub M, Gomori JM, Dotan S, Fellig Y, Shoshan Y. Rathke’s cleft cyst abscess. Pediatr Neurosurg. 2000. 33: 159-61
20. Jain KC, Varma A, Mahapatra AK. Pituitary abscess: A series of six cases. Br J Neurosurg. 1997. 11: 139-43
21. Joshi M, Gunawardena S, Goenka A, Ey E, Kumar G. Post COVID-19 lymphocytic hypophysitis: A rare presentation. Child Neurol Open. 2022. 9: 2329048X221103051
22. Komatsu F, Tsugu H, Komatsu M, Sakamoto S, Oshiro S, Fukushima T. Clinicopathological characteristics in patients presenting with acute onset of symptoms caused by Rathke’s cleft cysts. Acta Neurochir (Wien). 2010. 152: 1673-8
23. Liu F, Li G, Yao Y, Yang Y, Ma W, Li Y. Diagnosis and management of pituitary abscess: Experiences from 33 cases. Clin Endocrinol (Oxf). 2011. 74: 79-88
24. Lodha P, Srinivas PR, Danda VS, Rao GP. Rathke’s cleft cyst abscess-an unusual guest in the sella. Indian J Neurosurg. 2022. 11: 73-5
25. Misgar RA, Rasool A, Wani AI, Bashir MI. Central diabetes insipidus (Infundibuloneuro hypophysitis): A late complication of COVID-19 infection. J Endocrinol Invest. 2021. 44: 2855-6
26. Mrelashvili A, Braksick SA, Murphy LL, Morparia NP, Natt N, Kumar N. Chemical meningitis: A rare presentation of Rathke’s cleft cyst. J Clin Neurosci. 2014. 21: 692-4
27. Mungmunpuntipantip R, Wiwanitkit V. Pituitary apoplexy and COVID-19 vaccination. Med Clin (Engl Ed). 2022. 159: e11
28. Murvelashvili N, Tessnow A. A case of hypophysitis following immunization with the mRNA-1273 SARSCoV-2 vaccine. J Investig Med High Impact Case Rep. 2021. 9: 23247096211043386
29. Naama O, Gazzaz M, Boulahroud O, Elmoustarchid B. Infection of a Rathke cleft cyst: A rare cause of pituitary abscess. Surg Infect (Larchmt). 2014. 15: 358-60
30. Nishikawa T, Takahashi JA, Shimatsu A, Hashimoto N. Hypophysitis caused by Rathke’s cleft cyst. Case report. Neurol Med Chir (Tokyo). 2007. 47: 136-9
31. Nishioka H, Shibuya M, Haraoka J. Immunohistochemical study for IgG4-positive plasmacytes in pituitary inflammatory lesions. Endocr Pathol. 2010. 21: 236-41
32. Nonglait PL, Naik R, Raizada N. Hypophysitis after COVID-19 infection. Indian J Endocrinol Metab. 2021. 25: 255-6
33. Obenchain TG, Becker DP. Abscess formation in a Rathke’s cleft cyst. Case report. J Neurosurg. 1972. 36: 359-62
34. Pal R, Banerjee M. COVID-19 and the endocrine system: Exploring the unexplored. J Endocrinol Invest. 2020. 43: 1027-31
35. Que Y, Hu C, Wan K, Hu P, Wang R, Luo J. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int Rev Immunol. 2022. 41: 217-30
36. Sato M, Matsushima Y, Taguchi J, Matsumoto S, Tatsumi C, Ozaki M. A case of pituitary abscess caused by infection of Rathke’s cleft cyst. No Shinkei Geka. 1995. 23: 991-5 (In Japanese)
37. Shimatsu A, Oki Y, Fujisawa I, Sano T. Pituitary and stalk lesions (infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: An emerging clinical entity. Endocr J. 2009. 56: 1033-41
38. Sonntag VK, Plenge KL, Balis MS, Raudzens PA, Hodak JA, Clark RJ. Surgical treatment of an abscess in a Rathke’s cleft cyst. Surg Neurol. 1983. 20: 152-6
39. Takagi H, Iwama S, Sugimura Y, Takahashi Y, Oki Y, Akamizu T. Diagnosis and treatment of autoimmune and IgG4-related hypophysitis: Clinical guidelines of the Japan Endocrine Society. Endocr J. 2020. 67: 373-8
40. Takayasu T, Yamasaki F, Tominaga A, Hidaka T, Arita K, Kurisu K. A pituitary abscess showing high signal intensity on diffusion-weighted imaging. Neurosurg Rev. 2006. 29: 246-8
41. Tate MC, Jahangiri A, Blevins L, Kunwar S, Aghi MK. Infected Rathke cleft cysts: Distinguishing factors and factors predicting recurrence. Neurosurgery. 2010. 67: 762-9
42. Tsuda Y, Oguri T, Sakurai K, Watanabe T, Maeda N, Yuasa H. Intrasellar xanthogranuloma with abscess formation in a patient with Rathke’s cleft cyst. Rinsho Shinkeigaku. 2018. 58: 411-3 (In Japanese)
43. Uchiyama T, Sakai K, Asanuma M, Aoyama T, Hongo K. Pituitary abscess manifesting as meningitis and photophobia associated with Rathke’s cleft cyst in a child. Case report. Neurol Med Chir (Tokyo). 2011. 51: 455-9
44. Yang C, Wu H, Bao X, Wang R. Lymphocytic hypophysitis secondary to ruptured Rathke’s cleft cyst: Case report and literature review. World Neurosurg. 2018. 114: 172-7
45. Zerrouki D, Assarrar I, Rami I, Rouf S, Latrech H. Coronavirus as a trigger of lymphocytic hypophysitis in an adolescent girl: An exceptional case report. Int J Surg Case Rep. 2024. 115: 109218