- Department of Neurosurgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
- Department of Neurosurgery, King Khalid University Hospital, Riyadh, Saudi Arabia.
Correspondence Address:
Sarah Basindwah
Department of Neurosurgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia.
DOI:10.25259/SNI_633_2020
Copyright: © 2020 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdullah Alramadan1, Anwar Ul Haq1, Sarah Basindwah2, Essam Alshail1. Seizure outcome in moyamoya after indirect revascularization in pediatric patients: Retrospective study and literature review. 23-Feb-2021;12:73
How to cite this URL: Abdullah Alramadan1, Anwar Ul Haq1, Sarah Basindwah2, Essam Alshail1. Seizure outcome in moyamoya after indirect revascularization in pediatric patients: Retrospective study and literature review. 23-Feb-2021;12:73. Available from: https://surgicalneurologyint.com/surgicalint-articles/10608/
Abstract
Background: Moyamoya disease (MMD) is a unique cerebrovascular disorder characterized by progressive stenosis of anterior cerebral circulation. Moyamoya is not an uncommon disease in Saudi Arabia. Although a less common symptom of the disease, the incidence of seizure in MMD ranges from 6 to 30%. Indirect revascularization through Encephaloduroarteriosynangiosis technique is one of the surgical treatment options for MMD. In our cohort, we aim to evaluate seizure outcome in pediatric patients with moyamoya.
Methods: Eleven patients with seizure as primary presentation for MMD over a period of 10 years were included in the study. All patients underwent EDAS surgery. All patients underwent pre- and postoperative assessment of multiple factors contributing to seizure outcome. Patients were evaluated for surgery control clinically and radiologically.
Results: About 73% of MMD patients with seizures improved after EDAS surgery (P P
Conclusion: Patients with controlled seizure before surgery are more likely to be seizure free after intervention. Seizure outcome is favorable after indirect surgical revascularization in pediatric moyamoya patients.
Keywords: Encephaloduroarteriosynangiosis, Indirect revascularization, Moyamoya, Pediatric epilepsy
INTRODUCTION
Moyamoya disease (MMD) was first reported in Japan by Takeuchi and Shimizu in 1957 and then identified as a distinct disease by professor Suzuki in 1968.[
Moyamoya is not an uncommon disease in Saudi Arabia, multiple cases were reported for moyamoya syndrome secondary to protein S deficiency, cranial radiation, systemic lupus erythematosus, Fanconi anemia, and in association with diabetic ketoacidosis.[
It is estimated to represent 5.8–20% of stroke etiology in Saudi pediatrics patients.[
Symptoms accompanying MMD include stroke, seizure, and headaches.[
MATERIALS AND METHODS
Subjects
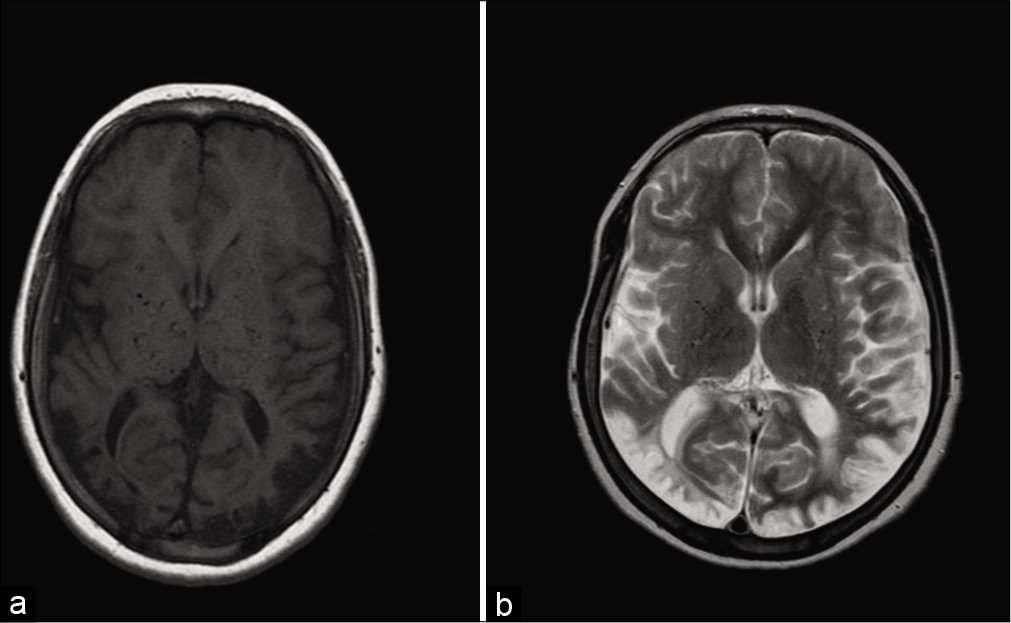
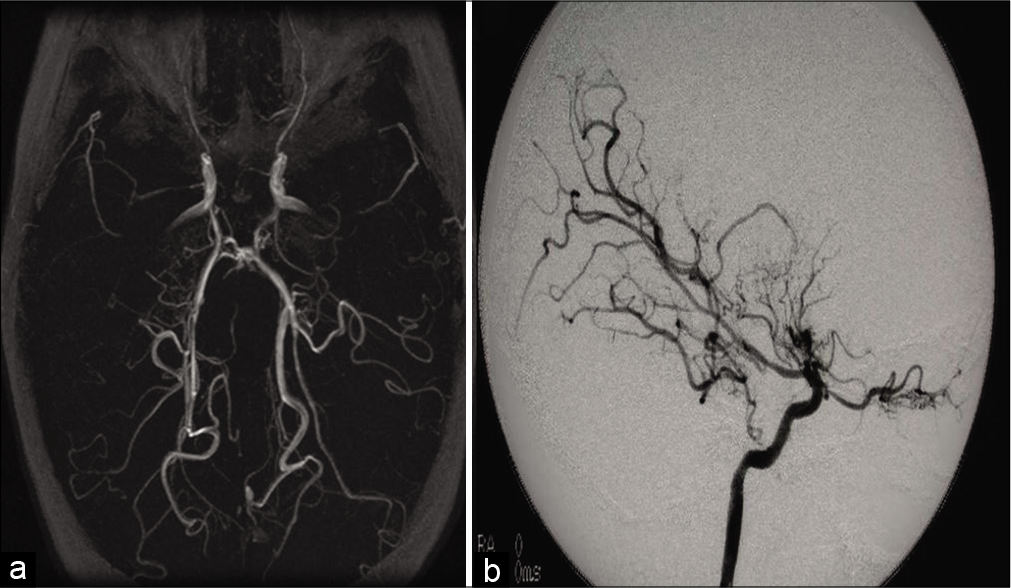
We selected patients who were diagnosed with moyamoya and underwent EDAS surgery at the Department of Neurosurgery, King Faisal Specialist Hospital and Research Center, Riyadh, between 2001 and 2011. The hospital research ethics board approved this study. Included patients had to be diagnosed with MMD with magnetic resonance imaging (MRI), magnetic resonance angiogram (MRA), and cerebral angiography findings of stenosis or occlusion in terminal intracranial ICA or proximal portions of anterior and middle cerebral artery with evidence of an abnormal vascular network. All included patients have seizure as a primary presenting symptom with postoperative follow-up period at our center of more than 5 years.
Retrospective review
We reviewed patient’s charts and clinical notes and images for patient’s sex, age at onset, age at first surgery, presentation of stroke and/ or seizure, developmental delay, cognitive impairment, seizure control, etiology, and radiological findings. Radiological findings were retrieved from multiple radiological reports reported by at least two experienced radiologists.
Clinical assessment and follow-up
Of all identified patients who underwent EDAS surgery and fit the inclusion criteria in the selected period, only patients who presented with seizures as a primary symptom and who were followed for more than 5 years were included in the study. Included patients were evaluated and managed by a pediatric epileptologist pre- and postintervention. We studied the etiology of moyamoya, either idiopathic or related to another pathology, presence, and type of stroke, pre- and postoperative seizure status, and the number of anti-epileptic drugs (AED) to control seizures. Before the intervention, all patients were evaluated by MRI, MRA, and cerebral angiogram [
Statistical analysis
We assessed patients on pre- and postsurgery number of AED used and seizure control. Dividing the patients into two groups: improved seizure control and not improved seizure control. We looked at different variables including radiological findings and surgical interventions. Variables were analyzed with binominal tests and Chi-square. P < 0.05 was considered statistically significant. Data were analyzed using SPSS for Windows, version 19.0.
RESULTS
A total of 22 patients were identified, 14 were having seizures at their initial presentation as a primary symptom, 11 patients were followed at least 5 years after surgery (5 males and 6 females) [
Patients demographics
The mean age of our patients with MMD at the time of disease onset was 6 years (from 11 months to 15 years). All 22 patients had ischemic strokes, except 1 patient diagnosed with sickle cell anemia and glucose-6-phosphate dehydrogenase deficiency who had a hemorrhagic stroke. Of all the 22 patients, 14 patients presented with seizures as the primary symptom of their stroke. Only four patients out of the 11 patients had both developmental delay and cognitive decline with no other etiology.
Etiology
Ten of the 22 patients had a secondary etiology to Moyamoya, seven of them are hematological disorders. Two patients were diagnosed with sickle cell disease and one patient carried sickle cell trait. Another patient had both sickle cell disease and G6PD deficiency. One patient with hypercoagulopathy, one patient with protein S deficiency, and one patient with alpha thalassemia. One patient had a craniopharyngioma that was resected and received radiotherapy before moyamoya diagnosis. One patient with glycogen storage disease and one patient with neurofibromatosis type 1.
Radiological findings
MRI, MRA, and cerebral angiography for all 22 patients showed bilaterality in 90% of patients before EDAS surgery. About 50% of patients showed posterior circulation involvement either at the time of diagnosis or with repeated images. ICA stenosis was noted in all patients, along with anterior and middle cerebral arteries.
All 22 patients had parenchymal changes on imaging, encephalomalacia, gliosis, and brain atrophy were noted. Most patients had large watershed infarcts. Degree of damage between patients with and without seizures was similar, involving large areas of the brain, sometimes a whole hemisphere.
Surgery status
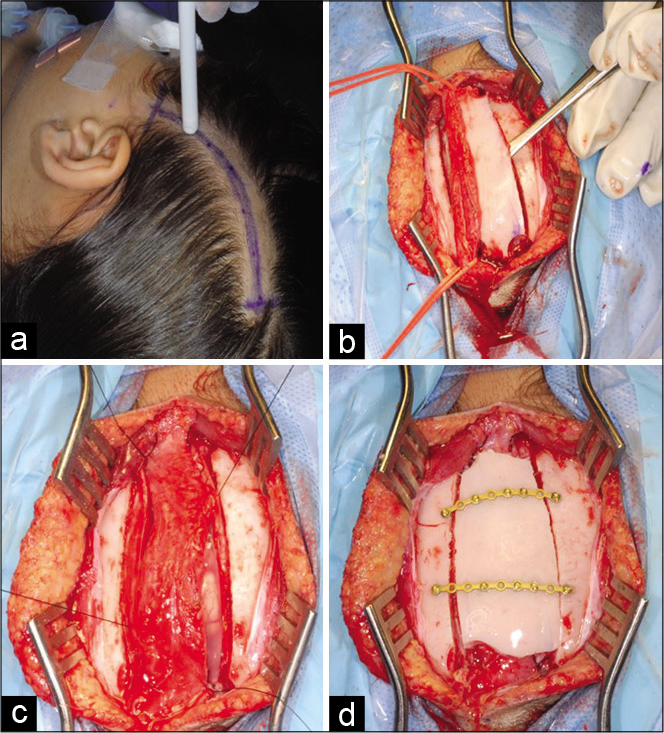
EDAS was performed after finding the superficial temporal artery using Doppler ultrasound. A small strip craniotomy is done to allow for easy placement of the artery that is then sutured under the dura and the bone is fixed back to allow for healthy formation of collaterals. The procedure was performed by the same surgeon in the same technique for all patients.
Eight of the 11 patients with epilepsy underwent bilateral EDAS surgery by the same surgeon, at least 3 months apart and at most 2 years apart. Three patients underwent unilateral surgery for the more severely affected side.
Seizure status
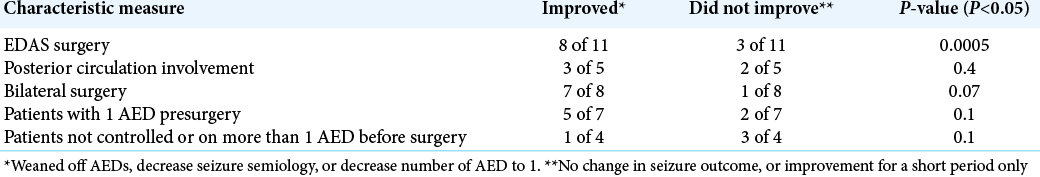
Of the 11 patients with primary presentation of seizure who were followed for at least 5 years, eight patients were controlled on AEDs (7 patients on 1 AED and 1 patient on 2 AED), seizure was not controlled with AED in three patients. Postoperatively, 55% of patients became seizure free (P = 0.02). Eight patients improved (either weaned off AED completely or decrease the number of AED used). Five of those are no longer on AED. Three patients did not improve (either no change or improved for a short period).
MRI findings of all 11 patients showed multiple infarcts around watershed areas, large areas of atrophy, and gliosis. Patients with posterior circulation involvement were 5 out of 11, three of those improved. Five out of six patients with no posterior circulation involvement improved [
Eight of 11 patients underwent bilateral EDAS surgery. Seven of those eight patients improved after surgeries. Three of 11 patients underwent unilateral EDAS surgery of the more affected side, one of those was weaned off AED.
DISCUSSION
Although seizure is one of the less common presentations of MMD, recent reports reveal different estimations of the number of pediatric patients presenting with seizure.[
In our study, approximately 63% (14 out of 22) of patients undergoing indirect revascularization surgery presented with seizure. Mikami et al. found five statistically significant predicting factors for epilepsy in MMD: cerebrovascular accident (CVA), lesion diameter in T2-weighted image in brain MRI, age, seizure onset after stroke and cortical involvement, and hemorrhagic type of stroke.[
The duration of epilepsy before revascularization was identified as an independent factor for future seizure recurrence in pediatric MMD.[
In a single-center study on long-term clinical outcome after EDAS surgery, 67% of patients showed excellent, complete resolution of preoperative symptoms (such as TIA or seizures) and no fixed neurological deficits, with a more favorable prognosis for patients presenting with symptoms at an older age (>3 years).[
All patients in our study underwent indirect revascularization (EDAS) surgery. In a study by Ma et al., there was no significant difference between direct and indirect revascularization in seizure outcome in pediatric moyamoya patients.[
In the previous reports, it was reasonable for MMD to be subdivided into four types depending on the presentation, including an epileptic type.[
CONCLUSION
Pediatric Moyamoya patients with epilepsy will significantly benefit from EDAS. Patients with controlled seizures before surgery are more likely to become seizure free post operatively.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Amro A, Schultz H. Moyamoya vasculopathy after cranial irradiation-a case report. Acta Oncol (Stockholm Sweden). 1995. 34: 261-3
2. Al-Hawsawi ZM, Al-Zaid MA, Barnawi AI, Yassine SM. Fanconi anemia associated with moyamoya disease in Saudi Arabia. Saudi Med J. 2015. 36: 233-5
3. Andeejani AM, Salih MA, Kolawole T, Gader AM, Malabarey TO, Al Damegh S. Moyamoya syndrome with unusual angiographic findings and protein C deficiency: Review of the literature. J Neurol Sci. 1998. 159: 11-6
4. Choi JI, Ha SK, Lim DJ, Kim SD. Differential clinical outcomes following encephaloduroarteriosynangiosis in pediatric moyamoya disease presenting with epilepsy or ischemia. Childs Nerv Syst. 2015. 31: 713-20
5. El Ramahi KM, Al Rayes HM. Systemic lupus erythematosus associated with moyamoya syndrome. Lupus. 2000. 9: 632-6
6. Emam AT, Ali AM, Babikr MA. Childhood stroke in Eastern Province, KSA: Pattern, risk factors, diagnosis and outcome. Acta Paediatr. 2009. 98: 1613-9
7. Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease).Research committee on spontaneous occlusion of the circle of willis (moyamoya disease) of the ministry of health and welfare Japan. Clin Neurol Neurosurg. 1997. 99: S238-40
8. Habib HS. Moyamoya disease presenting with ischemic stroke in association with diabetic ketoacidosis. J Pediatr Neurol. 2013. 11: 39-42
9. Kikuta K, Takagi Y, Arakawa Y, Miyamoto S, Hashimoto N. Absence epilepsy associated with moyamoya disease. Case report. J Neurosurg. 2006. 104: 265-8
10. Kimiwada T, Hayashi T, Shirane R, Tominaga T. Posterior cerebral artery stenosis and posterior circulation revascularization surgery in pediatric patients with moyamoya disease. J Neurosurg Pediatr. 2018. 21: 632-8
11. Ma Y, Zhao M, Zhang Q, Liu X, Zhang D, Wang S. Risk factors for epilepsy recurrence after revascularization in pediatric patients with moyamoya disease. J Stroke Cerebrovasc Dis. 2018. 27: 740-6
12. Matsushima Y, Fukai N, Tanaka K, Tsuruoka S, Inaba Y, Aoyagi M. A new surgical treatment of moyamoya disease in children: A preliminary report. Surg Neurol. 1981. 15: 313-20
13. Mikami T, Ochi S, Houkin K, Akiyama Y, Wanibuchi M, Mikuni N. Predictive factors for epilepsy in moyamoya disease. J Stroke Cerebrovasc Dis. 2015. 24: 17-23
14. Moritake K, Handa H, Yonekawa Y, Taki W, Okuno T. Follow-up study on the relationship between age at onset of illness and outcome in patients with moyamoya disease. No Shinkei Geka. 1986. 14: 957-63
15. Nakase H, Ohnishi H, Touho H, Miyamoto S, Watabe Y, Itoh T. Long-term follow-up study of “epileptic type” moyamoya disease in children. Neurol Med Chir (Tokyo). 1993. 33: 621-4
16. Pines AR, Rodriguez D, Bendok BR, Dhamija R. Clinical characteristics of moyamoya angiopathy in a pediatric cohort. J Child Neurol. 2020. 35: 389-92
17. Riordan CP, Storey A, Cote DJ, Smith ER, Scott RM. Results of more than 20 years of follow-up in pediatric patients with moyamoya disease undergoing pial synangiosis. J Neurosurg Pediatr. 2019. p. 1-7
18. Salih MA, Murshid WR, Al-Salman MM, Abdel-Gader AG, Al-Jarallah AA, Alorainy IA. Moyamoya syndrome as a risk factor for stroke in Saudi children. Novel and usual associations. Saudi Med J. 2006. 27: S69-80
19. Suzuki J, Kodama N. Moyamoya disease-a review. Stroke. 1983. 14: 104-9
20. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. 1969. 20: 288-99
21. Suzuki J.editors. Moyamoya Disease. Berlin: Springer; 1986. p.
22. Zhang Y, Bao XY, Duan L, Yang WZ, Li DS, Zhang ZS. Encephaloduroarteriosynangiosis for pediatric moyamoya disease: Long-term follow-up of 100 cases at a single center. J Neurosurg Pediatr. 2018. 22: 173-80