- Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga,
- Department of Neurosurgery, Faculty of Medicine, Universitas Airlangga, / Dr. Soetomo General Academic Hospital, Surabaya, East Java, Indonesia,
- Stem Cell Research and Development Center, Universitas Airlangga,
- Department of Microbiology, Virology and Immunology Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga,
- Biomedical Engineering Study Program, Department of Physics, Faculty of Science and Technology, Universitas Airlangga, Surabaya 60115, Indonesia.
Correspondence Address:
Asra Al Fauzi, Department of Neurosurgery, Faculty of Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia.
DOI:10.25259/SNI_470_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nur Setiawan Suroto1,2, Fedik Abdul Rantam3,4, Asra Al Fauzi2, Prihartini Widiyanti5, Agus Turchan2, Vega Pangaribuan2. Selection criteria for patch angioplasty material in carotid endarterectomy. 19-Aug-2022;13:362
How to cite this URL: Nur Setiawan Suroto1,2, Fedik Abdul Rantam3,4, Asra Al Fauzi2, Prihartini Widiyanti5, Agus Turchan2, Vega Pangaribuan2. Selection criteria for patch angioplasty material in carotid endarterectomy. 19-Aug-2022;13:362. Available from: https://surgicalneurologyint.com/surgicalint-articles/11816/
Abstract
Background: Carotid endarterectomy (CEA) with patch angioplasty has been favored due to its lower reoccurrence of restenosis compared to primary CEA. There are multiple types of patch angioplasty material available. However, selection of patch material is based on uncertain criteria. The aim of this study is to determine the ideal criteria for selecting the best patch material for CEA.
Methods: We conducted a comprehensive literature search for studies that describe the ideal criteria for selecting patch material for CEA. We compiled all of the criteria mentioned into one table and selecting the criteria which were most frequently mentioned with a simple scoring system.
Results: A total of 65 studies out of 784 studies were assessed for its full-text eligibility. Thus, we found 23 studies that were eligible for analysis. There are 22 ideal criteria that were mentioned in the analyzed studies. We grouped these criteria into physical characteristics, safety, contribution to hemodynamic, contribution in tissue healing, economic aspect, and ability to prevent postsurgical complication. We proposed 10 ideal criteria for guiding vascular surgeon in selecting the best patch angioplasty material.
Conclusion: To this day, no material has been discovered which meets all ten criteria. This study’s proposed ideal criteria serve as the foundation for the creation of the best patch angioplasty material.
Keywords: Carotid endarterectomy, Ideal criteria, Material, Patch angioplasty
INTRODUCTION
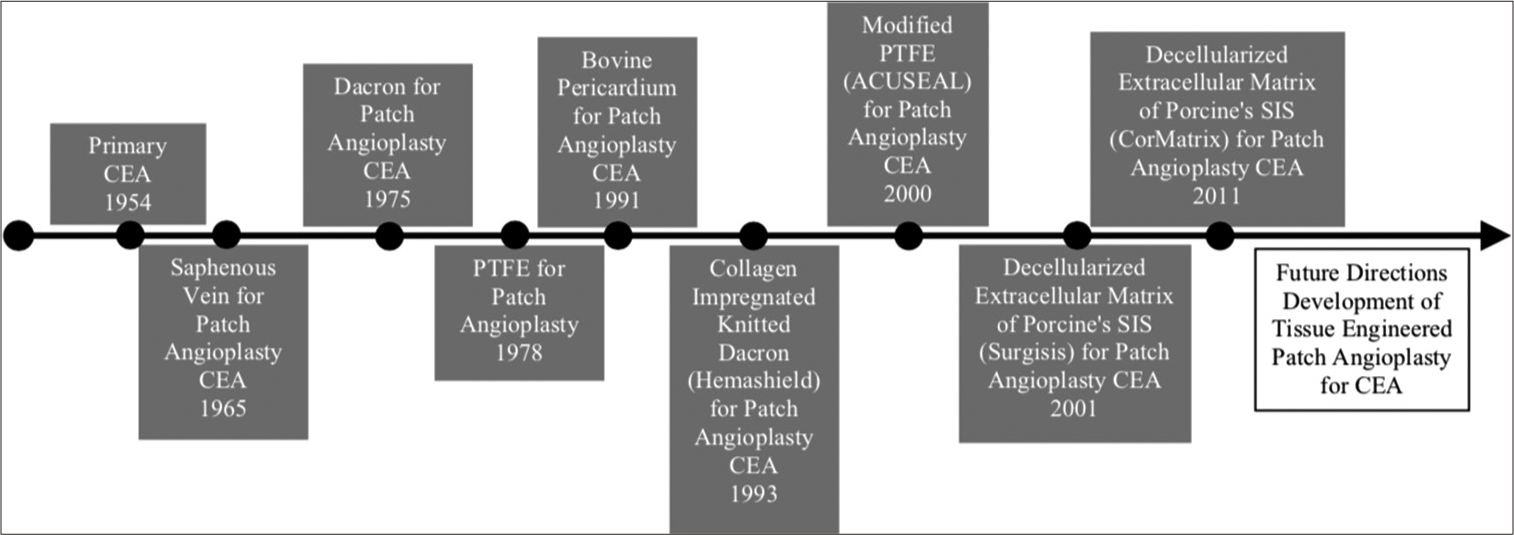
Carotid endarterectomy (CEA) remains a mainstay treatment for carotid stenosis since it was first conducted in 1954 by Eastcott et al.[
There are several materials that can be used for patch angioplasty, those are autologous vein, prosthetic patches (polytetrafluoroethylene [PTFE], Dacron, polyurethane, and polyester), and biologic patches (bovine pericardium and decellularized extracellular matrix). However, the outcome of different patch materials seemed to be similar in terms of short- and long-term results.[
Vascular surgeons have not been able to identify the specific and clear guidance in choosing materials for patch angioplasty. This systematic review aimed to find the ideal criteria for patch angioplasty in CEA and whether the materials that were studied have fulfilled the ideal criteria or not.
MATERIALS AND METHODS
Literature search
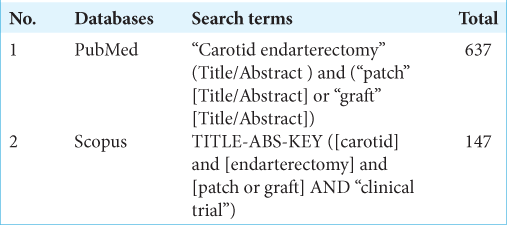
The search strategy was in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidance. We performed a literature search in PubMed and SCOPUS in June 8, 2021. The keywords that were used are carotid AND Endarterectomy AND Patch. The detailed search terms can be seen in the Supplementary
Eligibility criteria
The articles included in this research met the following criteria: (1) experimental procedure in human carotid artery, (2) mentioned the ideal criteria for selecting patch angioplasty material, (3) revealed the source of the patch material, (4) parameter used to evaluate complications, (5) examined a single type of material during study, and (6) the full text is available in English. Any duplicated unavailable full texts, review papers, case reports, case series, or abstract-only papers are not included in our review.
Data extraction
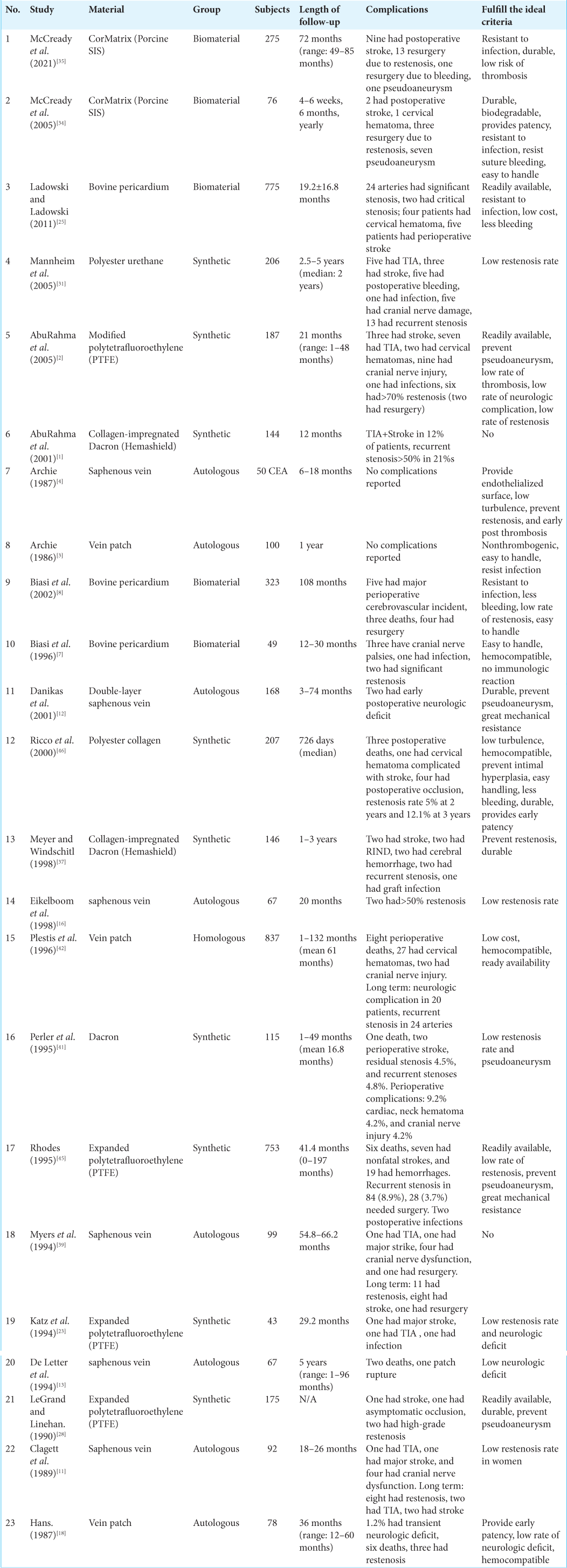
Two reviewers blind to each other independently extracted the relevant data from the included studies. Any disagreements will be resolved through discussion among the two reviewers. The data that were obtained from the full-text review are author, year of publication, number of subjects, and material that were used, ideal criteria for patch angioplasty material, time of follow-up, complications observed, and fulfillment of the ideal criteria.
RESULTS
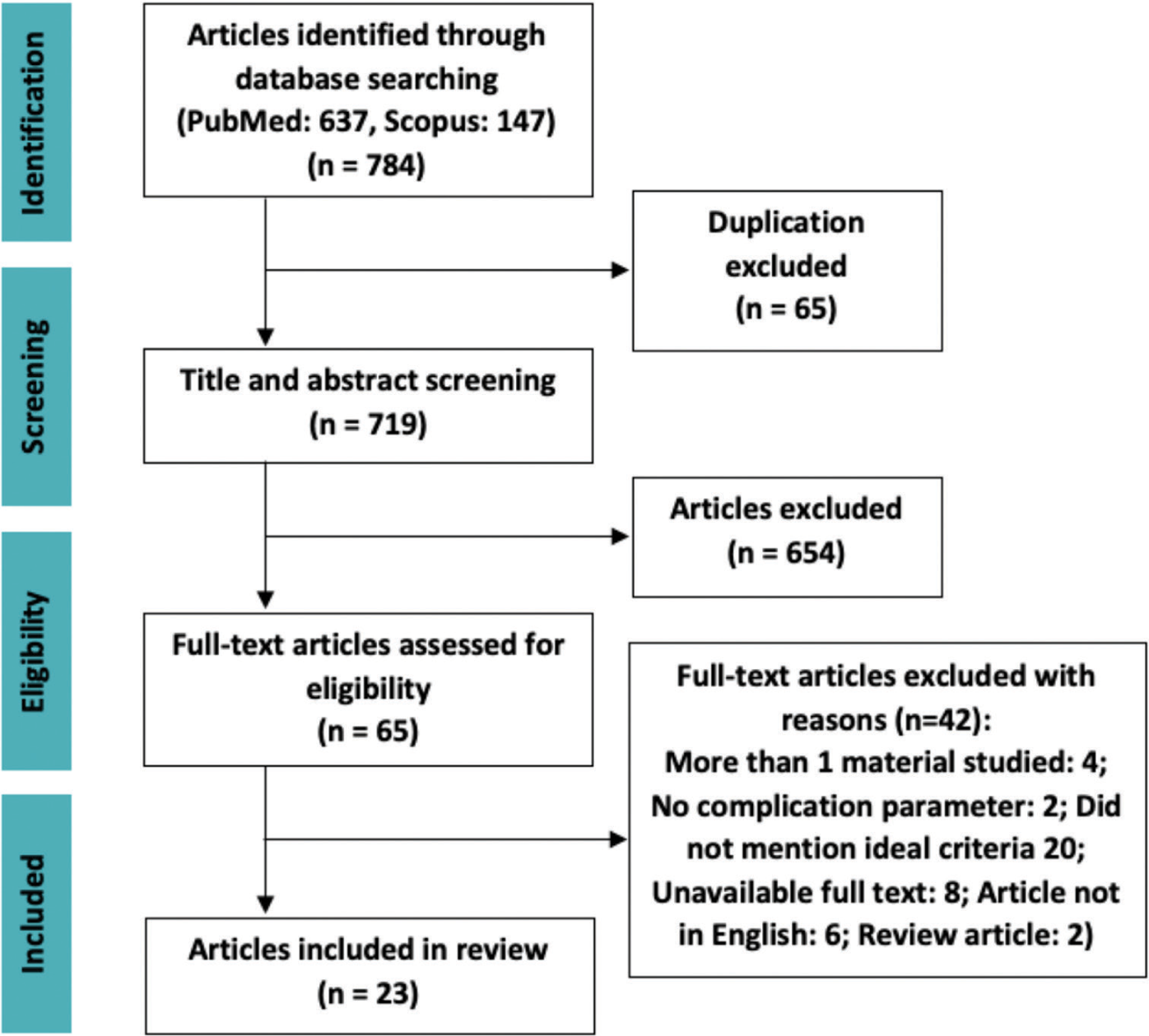
The search engine PubMed and SCOPUS provide a total of 784 articles. Duplications were found using EndNote X9, excluding 65 articles. Screening of title and abstract by our reviewers excluded 654 articles. Additional 42 full-text articles were excluded due to its inability to fulfill the eligibility criteria; thus, 23 articles were included in our study [
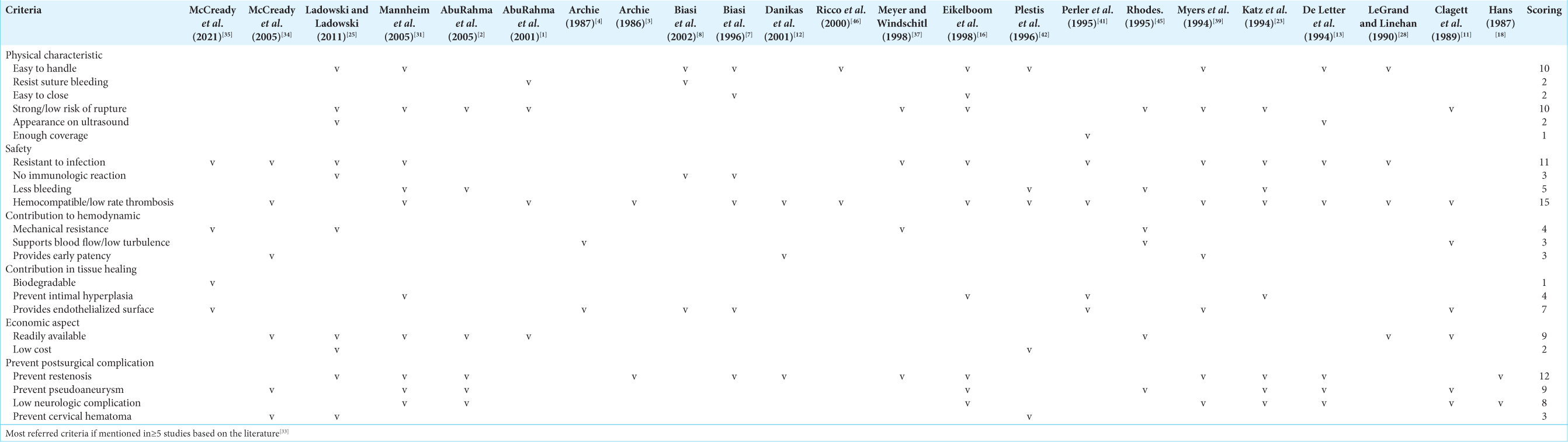
The simple scoring system conducted in our study was derived from a study by Bolly et al., which able to determine the ideal criteria for duraplasty material.[
We compiled all of the ideal criteria and grouped those criteria into physical characteristics, safety, contribution to hemodynamic, contribution in tissue healing, economic aspect, and ability to prevent postsurgical complication. All of the findings of ideal criteria were listed into a table, and the dominant ideal criteria should have been mentioned in at least five papers. There are 10 criteria that are considered as the most dominant and the most ideal ones (mentioned ≥5 times), those are easy to handle, durable/ low risk of rupture, resistant to infection, less bleeding, hemocompatible, provide endothelialized surface, readily available, prevent restenosis, prevent pseudoaneurysm, and low neurologic complication [
DISCUSSION
CEA for carotid artery stenosis is indicated by European Society of Cardiology for symptomatic patients with stenosis >50%, while asymptomatic patients indicated to be intervened when stenosis >60% and had high long-term risk of stroke.[
Our review found that the main ideal criteria are as follows: easy to handle, durable/low risk of rupture, resistant to infection, less bleeding, hemocompatible, provides endothelialization, readily available, prevent restenosis, prevent pseudoaneurysm, and provide low neurologic complication. At present, there is no material that is able to fulfill all of those ideal criteria.
Historically, autologous veins are being favored for conducting patch angioplasty CEA, since it provides endothelialized surface, similar to the surrounding arterial tissue, easy handling, and low risk of infection. However, its apparent disadvantages, such as the extended operation duration, the probability of wound complications at the harvest site, and the likelihood of rupture and pseudoaneurysm, cannot be overlooked.[
In our review, the most frequently selected ideal criteria were hemocompatibility, since a thrombogenic substance might induce postoperative stroke, resulting in severe morbidity for the patients. After implantation of vascular patch, plasma protein will be absorbed immediately; they contain binding sites for integrin receptors, which can be found on platelets and many cells. Platelets may become activated as a result of adhesion to these receptors.[
Resistance to infection is also important in providing a safe patch angioplasty CEA. Braided sutures in the synthetic patches can trap bacteria in their interstices, thus providing nidus of infection. Synthetic patches such as PTFE have been modified using monofilament sutures to eliminate this risk. However, the number of infections in synthetic patches is still considerably higher than other materials.[
Limitations
Our study focused on patch material as a main contributor for a successful CEA procedure. However, we cannot disregard other factors that may contribute to post-CEA complications. One of our proposed ideal criteria and the main goal of any carotid artery stenosis intervention are prevention of restenosis. Although patch material is knowingly affected restenosis rate, arterial intervention itself can promote restenosis.[
Future directions
Historically, the characteristics of patch angioplasty which were desired are those with low risk of pseudoaneurysm, prevent rupture, and readily available, since autologous vein cannot provide these characteristics. Synthetic materials and biomaterials are being developed to solve these problems. However, with the use of these foreign materials, several problems ensued. Synthetic materials such as PTFE and Dacron are still at risk for infection, can be thrombogenic, and had prolonged hemostasis time.[
Development of tissue-engineered patch angioplasty is hoped to provide a patch material that can fulfill all of the proposed ideal criteria. The advantage of tissue engineered patch angioplasty is providing a material that integrates with host tissue and has characteristic like a native vessel; thus, the desired ideal criteria can be achieved. One of the most promising tissue-engineered vascular tissues is by nanofiber composites, which combine natural and synthetic polymers.[
CONCLUSION
This review provided 10 ideal criteria that can be used to help surgeons to choose a material for patch angioplasty CEA. Up until this day, material than possesses all of the 10 criteria has not yet been found. The ideal criteria in this review serve as foundation in the development of the ideal patch angioplasty material for CEA procedure.
Disclosures
The authors have no relevant financial or nonfinancial interest to disclose.
Declaration of patient consent
Patients’ consent not required as there are no patients’ in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY
References
1. AbuRahma AF, Robinson PA, Hannay RS, Hudson J, Cutlip L. Prospective controlled study of carotid endarterectomy with hemashield patch: Is it thrombogenic?. Vasc Surg. 2001. 35: 167-74
2. AbuRahma AF, Stone PA, Welch CA, Hofeldt MJ, Hass SM, Perry W. Prospective study of carotid endarterectomy with modified polytetrafluoroethylene (ACUSEAL) patching: Early and late results. J Vasc Surg. 2005. 41: 789-93
3. Archie JP. Prevention of early restenosis and thrombosis-occlusion after carotid endarterectomy by saphenous vein patch angioplasty. Stroke. 1986. 17: 901-5
4. Archie JP. The geometry and mechanics of saphenous vein patch angioplasty after carotid endarterectomy. Tex Heart Inst J. 1987. 14: 395-400
5. Avrahami I, Raz D, Bash O. Biomechanical aspects of closing approaches in postcarotid endarterectomy. Comput Math Methods Med. 2018. 2018: 4517652
6. Bekelis K, Moses Z, Missios S, Desai A, Labropoulos N. Indications for treatment of recurrent carotid stenosis. Br J Surg. 2013. 100: 440-7
7. Biasi GM, Mingazzini P, Baronio L, Sampaolo A. Processed bovine pericardium as patch angioplasty for carotid endarterectomy: A preliminary report. Cardiovasc Surg. 1996. 4: 848-52
8. Biasi GM, Sternjakob S, Mingazzini PM, Ferrari SA. Nine-year experience of bovine pericardium patch angioplasty during carotid endarterectomy. J Vasc Surg. 2002. 36: 271-7
9. Bonati LH, Gregson J, Dobson J, McCabe DJH, Nederkoorn PJ, van der Worp HB. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the International Carotid Stenting Study (ICSS): Secondary analysis of a randomised trial. Lancet Neurol. 2018. 17: 587-96
10. Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg. 2004. 40: 1126-35
11. Clagett GP, Patterson CB, Fisher DF, Fry RE, Eidt JF, Humble TH. Vein patch versus primary closure for carotid endarterectomy. A randomized prospective study in a selected group of patients. J Vasc Surg. 1989. 9: 213-23
12. Danikas D, Schmeler KM, Ginalis EM, Stratoulias C, Shorten SM, Constantinopoulos G. Double-layer everted saphenous vein patch angioplasty for carotid endarterectomy. Vasc Surg. 2001. 35: 259-61
13. De Letter JA, Moll FL, Welten RJ, Eikelboom BC, Ackerstaff RG, Vermeulen FE. Benefits of carotid patching: A prospective randomized study with long-term follow-up. Ann Vasc Surg. 1994. 8: 54-8
14. Eastcott HH, Pickering GW, Rob CG. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954. 267: 994-6
15. Edenfield L, Blazick E, Eldrup-Jorgensen J, Healey C, Bloch P, Hawkins R. Outcomes of carotid endarterectomy in the vascular quality initiative based on patch type. J Vasc Surg. 2020. 71: 1260-7
16. Eikelboom BC, Ackerstaff RG, Hoeneveld H, Ludwig JW, Teeuwen C, Vermeulen FEE. Benefits of carotid patching: A randomized study. J Vasc Surg. 1988. 7: 240-7
17. Gunn J, Zhang M. Polyblend nanofibers for biomedical applications: Perspectives and challenges. Trends Biotechnol. 2010. 28: 189-97
18. Hans SS, Girishkumar H, Hans B. Venous patch grafts and carotid endarterectomy. A critical appraisal. Arch Surg. 1987. 122: 1134-8
19. Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: A meta-analysis of pooled patient data from four randomised trials. Lancet (London, England). 2016. 387: 1305-11
20. Huizing E, Vos CG, Hulsebos RG, van den Akker PJ, Borst GJ, Ünlü Ç. Patch angioplasty or primary closure following carotid endarterectomy for symptomatic carotid artery stenosis. Surg J (N Y). 2018. 4: e96-101
21. Huizing E, Vos CG, van den Akker PJ, Schreve MA, de Borst GJ, Ünlü Ç. A systematic review of patch angioplasty versus primary closure for carotid endarterectomy. J Vasc Surg. 2019. 69: 1962-74.e4
22. Kamenskiy AV, Mactaggart JN, Pipinos II, Gupta PK, Dzenis YA. Hemodynamically motivated choice of patch angioplasty for the performance of carotid endarterectomy. Ann Biomed Eng. 2013. 41: 263-78
23. Katz D, Snyder SO, Gandhi RH, Wheeler JR, Gregory RT, Gayle RG. Long-term follow-up for recurrent stenosis: A prospective randomized study of expanded polytetrafluoroethylene patch angioplasty versus primary closure after carotid endarterectomy. J Vasc Surg. 1994. 19: 198-203
24. Knight BC, Tait WF. Dacron patch infection following carotid endarterectomy: A systematic review of the literature. Eur J Vasc Endovasc Surg. 2009. 37: 140-8
25. Ladowski JM, Ladowski JS. Retrospective analysis of bovine pericardium (Vascu-Guard) for patch closure in carotid endarterectomies. Ann Vasc Surg. 2011. 25: 646-50
26. Lal BK, Beach KW, Roubin GS, Lutsep HL, Moore WS, Malas MB. Restenosis after carotid artery stenting and endarterectomy: A secondary analysis of CREST, a randomised controlled trial. Lancet Neurol. 2012. 11: 755-63
27. Leal BBJ, Wakabayashi N, Oyama K, Kamiya H, Braghirolli DI, Pranke P. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front Cardiovasc Med. 2020. 7: 592361
28. LeGrand DR, Linehan RL. The suitability of expanded PTFE for carotid patch angioplasty. Ann Vasc Surg. 1990. 4: 209-12
29. Li X, Guo Y, Ziegler KR, Model LS, Eghbalieh SD, Brenes RA. Current usage and future directions for the bovine pericardial patch. Ann Vasc Surg. 2011. 25: 561-8
30. Malas M, Glebova NO, Hughes SE, Voeks JH, Qazi U, Moore WS. Effect of patching on reducing restenosis in the carotid revascularization endarterectomy versus stenting trial. Stroke. 2015. 46: 757-61
31. Mannheim D, Weller B, Vahadim E, Karmeli R. Carotid endarterectomy with a polyurethane patch versus primary closure: A prospective randomized study. J Vasc Surg. 2005. 41: 403-7
32. Marsman MS, Wetterslev J, Jahrome AK, Gluud C, Moll FL, Keus F. Carotid endarterectomy with patch angioplasty versus primary closure in patients with symptomatic and significant stenosis: A systematic review with meta-analyses and trial sequential analysis of randomized clinical trials. Syst Rev. 2021. 10: 139
33. Masang Ban Bolly H, Faried A, Laurens Jembise T, Fuad Wirakusumah F. The ideal selection criteria for duraplasty material in brain surgery: A review. Interdiscip Neurosurg Adv Tech Case Manag. 2020. 22: 100800
34. McCready RA, Hodde J, Irwin RJ, Coffey AC, Divelbiss JL, Bryant MA. Pseudoaneurysm formation in a subset of patients with small intestinal submucosa biologic patches after carotid endarterectomy. J Vasc Surg. 2005. 41: 782-8
35. McCready RA, Kiell CS, Chugh AR, Rapp BM, Webb TH, Barksdale A. Long-term results with cormatrix extracellular matrix patches after carotid endarterectomy. J Surg Res. 2021. 262: 21-6
36. Messas E, Goudot G, Halliday A, Sitruk J, Mirault T, Khider L. Management of carotid stenosis for primary and secondary prevention of stroke: State-of-the-art 2020: A critical review. Eur Heart J Suppl. 2020. 22: M35-42
37. Meyer FB, Windschitl WL. Repair of carotid endarterectomy with a collagen-impregnated fabric graft. J Neurosurg. 1998. 88: 647-9
38. Muto A, Nishibe T, Dardik H, Dardik A. Patches for carotid artery endarterectomy: Current materials and prospects. J Vasc Surg. 2009. 50: 206-13
39. Myers SI, Valentine RJ, Chervu A, Bowers BL, Clagett GP. Saphenous vein patch versus primary closure for carotid endarterectomy: Long-term assessment of a randomized prospective study. J Vasc Surg. 1994. 19: 15-22
40. Obregón R, Ramón-Azcón J, Ahadian S, Ramalingam M, Ramakrishna S.editors. Nanofiber Composites in Blood Vessel Tissue Engineering. Sawston, United Kingdom: Woodhead Publishing; 2017. p. 483-506
41. Perler BA, Ursin F, Shanks U, Williams GM. Carotid Dacron patch angioplasty: Immediate and long-term results of a prospective series. Cardiovasc Surg. 1995. 3: 631-6
42. Plestis KA, Kantis G, Haygood K, Earl N, Howell JF. Carotid endarterectomy with homologous vein patch angioplasty: A review of 1006 cases. J Vasc Surg. 1996. 24: 109-19
43. Ren S, Li X, Wen J, Zhang W, Liu P. Systematic review of randomized controlled trials of different types of patch materials during carotid endarterectomy. PLoS One. 2013. 8: e55050
44. Rerkasem K, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. Asian J Surg. 2011. 34: 32-40
45. Rhodes VJ. Expanded polytetrafluoroethylene patch angioplasty in carotid endarterectomy. J Vasc Surg. 1995. 22: 724-30
46. Ricco JB, Bouin-Pineau MH, Demarque C, Suy R, Kerdiles Y, Garbé JF. The role of polyester patch angioplasty in carotid endarterectomy: A multicenter study. Ann Vasc Surg. 2000. 14: 324-33
47. Sarkar S, Sales KM, Hamilton G, Seifalian AM. Addressing thrombogenicity in vascular graft construction. J Biomed Mater Res B Appl Biomater. 2007. 82: 100-8
48. Stone PA, Srivastava M, Campbell JE, Mousa AY, Hass SH, Kazmi H. A 10-year experience of infection following carotid endarterectomy with patch angioplasty. J Vasc Surg. 2011. 53: 1473-7
49. Texakalidis P, Giannopoulos S, Charisis N, Giannopoulos S, Karasavvidis T, Koullias G. A meta-analysis of randomized trials comparing bovine pericardium and other patch materials for carotid endarterectomy. J Vasc Surg. 2018. 68: 1241-56.e1