- Department of Neurosurgery, National Neuroscience Institute, Singapore
Correspondence Address:
Jia Xu Lim
Department of Neurosurgery, National Neuroscience Institute, Singapore
DOI:10.4103/2152-7806.163819
Copyright: © 2015 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Lim JX, King N, Low S, Ng WH. Serum procalcitonin is a marker for prediction of readmission from an intermediate care to an acute care hospital in neurosurgical patients. Surg Neurol Int 28-Aug-2015;6:144
How to cite this URL: Lim JX, King N, Low S, Ng WH. Serum procalcitonin is a marker for prediction of readmission from an intermediate care to an acute care hospital in neurosurgical patients. Surg Neurol Int 28-Aug-2015;6:144. Available from: http://surgicalneurologyint.com/surgicalint_articles/serum-procalcitonin-is-a-marker-for-prediction-of-readmission-from-an-intermediate-care-to-an-acute-care-hospital-in-neurosurgical-patients/
Abstract
Background:Readmission of patients to acute hospitals contributes significantly toward inefficient utilization of healthcare resources, with studies quoting up to 90% being preventable. We aim to report and analyze the factors involved in the readmission of neurosurgical patients who had been previously transferred to an intermediate step-down care facility, and explore possible predictive markers for such readmissions.
Methods:We conducted a retrospective analysis of all 129 neurosurgical patients who were transferred from out acute tertiary hospital to an intermediate care facility. The cases were segregated into those who were readmitted and those who were not readmitted back to our acute center. The demographic data, clinical features, diagnoses, treatment modalities, pretransfer laboratory findings, and inpatient complications were compared with readmission rate.
Results:There were 23 patients (17.8%) who were readmitted to our acute hospital. The most common causes of readmission was infection (n = 12, 52.2%). We found a statistically significant correlation between the higher pretransfer procalcitonin levels with the readmission of our patients (P = 0.037). There was also a significant difference noted between ethnic groups (P = 0.026) and having no complications of disease or treatment (P = 0.008), with readmission.
Conclusion:Procalcitonin is a pro-hormone known to correlate with infection and poor neurological status. We have found that its serum values correlate significantly with the readmission rates of neurosurgical patients in our study. We postulate that by ensuring normality in procalcitonin levels prior to transfer to an intermediate care facility, potentially half of neurosurgical readmissions can be prevented.
Keywords: Intermediate care, neurosurgery, procalcitonin, readmission
INTRODUCTION
Neurosurgical patients make up a significant proportion of long-term hospital inpatients in Singapore. They are admitted for a myriad of problems, ranging from intracranial hemorrhage to spinal injuries. It is well-established that neurosurgical patients, especially those suffering from traumatic brain injuries, require long-term care and rehabilitation.[
Intermediate care is defined as health-related care and service to individuals who do not require the degree of care that acute hospitals or skilled nursing facilities provide, but because of their physical or mental condition require care and services above the level of room and board.[
Among patients admitted into intermediate care, there is a fraction requiring single or multiple readmissions back into the acute hospital. This places an unnecessary burden on the healthcare system and increases healthcare costs, which is one of the main challenges of the healthcare system.[
Extensive studies into the American medical insurance system, “Medicare,” have put the impact of readmissions into perspective. Medicare studies have revealed a 19–22% readmission rate within 30 days, and up to 34% within 90 days.[
In our literature review, there were few articles describing predictive markers for readmission. In a study of colorectal patients, factors identified to be associated with readmissions included the female gender, prednisolone use, long operative duration, major postoperative complications, and surgical site infections.[
Procalcitonin, composing of 116 amino acids, is a peptide precursor of the hormone calcitonin. It is produced by the parafollicular cells of the thyroid and neuroendocrine cells of the lungs and intestines. Procalcitonin has been found in many studies to be elevated in patients with sepsis and severe infections,[
We aim to report and analyze the factors involved in the readmission of neurosurgical patients over a 2½-year period, and to explore possible predictive markers for readmission in an attempt to prevent or decrease the incidence of readmissions.
MATERIALS AND METHODS
Institutional review board approval was obtained from the National Health Group Domain Specific Review Board. We retrospectively analyzed all 129 case records of consecutive neurosurgical patients transferred to the intermediate care facility from the Department of Neurosurgery, National Neuroscience Institute, Tan Tock Seng Hospital campus, a 1200-bed acute tertiary hospital in Singapore, between the August 5, 2008 and May 28, 2011. The patients in the study were stratified into those who were discharged from intermediate care to either home or other long-term care facilities such as nursing homes, and those who required readmission from the intermediate care facility back to our acute tertiary hospital. In patients requiring multiple readmissions, only data obtained from the first readmission was used for analysis.
Demographic data collected from our patients include age at presentation, gender, ethnicity, previous cerebral, and vascular comorbidities (such as strokes, diabetes mellitus, hypertension, and hyperlipidemia), as well as premorbid status including ambulatory ability. The relevant clinical findings, laboratory results, diagnoses, and treatment underwent by the patient (conservative vs. surgical) were also collected. Clinical findings consist of vital signs and determinants of neurological status on admission, which comprised of the Glasgow Coma Scale (GCS) score and clinical manifestations of neurological deficits. Neurological deficits include, but are not limited to, weakness, sensory loss, and pupillary dilatation. Pretransfer laboratory results collected include full blood count, renal panel, C-reactive protein, procalcitonin, and albumin.
We also analyzed the complications were developed in patient such as infection and seizures. The duration of stay in the acute tertiary hospital and intermediate care facility was respectively analyzed and correlated with the incidence of readmission.
We used the following criteria to determine suitability for patient's transfer to the intermediate care facility: Stable vital parameters, afebrile for ≥24 h, and no further indication for neurosurgical or medical intervention as determined by the managing neurosurgeon.
All data were entered into a computerized database system for descriptive analysis, and analyzed with IBM SPSS Statistics (version 19, IBM Corp., New York, USA). Statistical tests used were Student's t-test, Pearson Chi-square goodness-of-fit test, and Fisher's exact test when deemed appropriate. Post-hoc analysis was used to compare adjusted standardized residuals to the cut-off of 2, for associations between ethnicity and readmissions. A P < 0.05 was considered to indicate statistical significance.
RESULTS
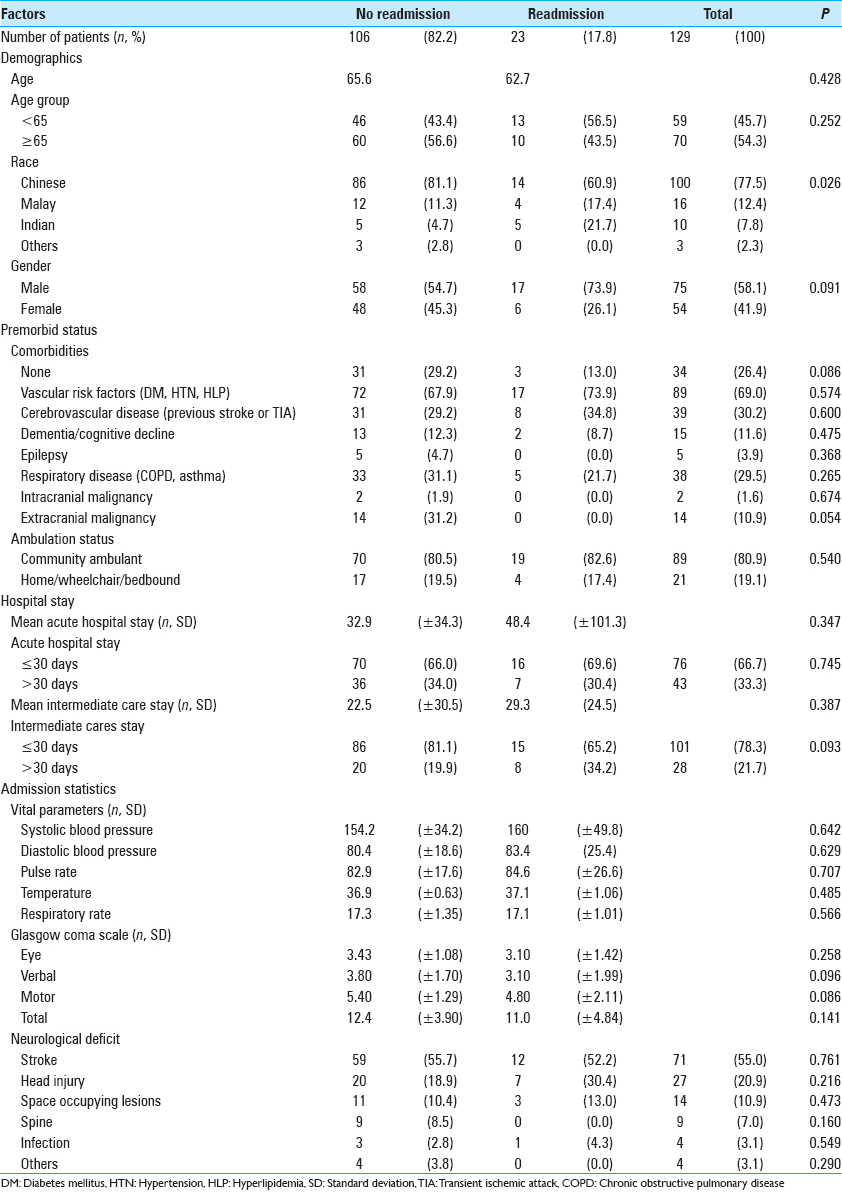
Our study included 129 neurosurgical patients transferred from our acute tertiary center. There were 75 males (58.1%) and 54 females (41.9%). The majority of the patients were Chinese (n = 100, 77.5%), followed by Malay (n = 16, 12.4%). The mean age of neurosurgical patients that was transferred was 65.1 years, and most patients were ≥65 years old (n = 70, 54.2%).
Of the 129 patients studied, 106 patients (82.2%) did not require readmission, and 23 patients (17.8%) required readmission. The detailed demographic profile of our patients stratified into the two populations is listed in
We found a statistically significant correlation between ethnicity and requirement of readmission (P = 0.026) using the Pearson Chi-square test. Using post-hoc analysis, we observed that there were fewer than expected readmissions in Chinese subjected (adjusted residual = −2.1). Correlation between patients with prior extracranial malignancies and readmission rates approached significance (P = 0.054). However, there were no significant correlations noted between readmission back to our acute tertiary center and the other demographic factors such as age, premorbid ambulatory status, and the presence of significant comorbidities. There was also no significant correlation between readmission rates and the length of stay in our acute hospital (P = 0.347) and our intermediate care facility (P = 0.093). Vitals signs, GCS, and neurological deficits on the admission of patients who were not readmitted were not appreciably different from those who were readmitted.
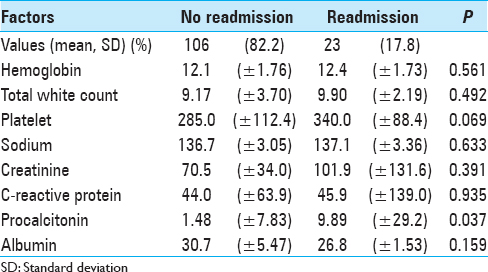
We found a significant correlation between high pretransfer procalcitonin levels and readmission rates (P = 0.037). There were otherwise no other laboratory markers that correlated significantly with readmission rates. Pretransfer biochemical markers stratified by the presence of readmission are presented in
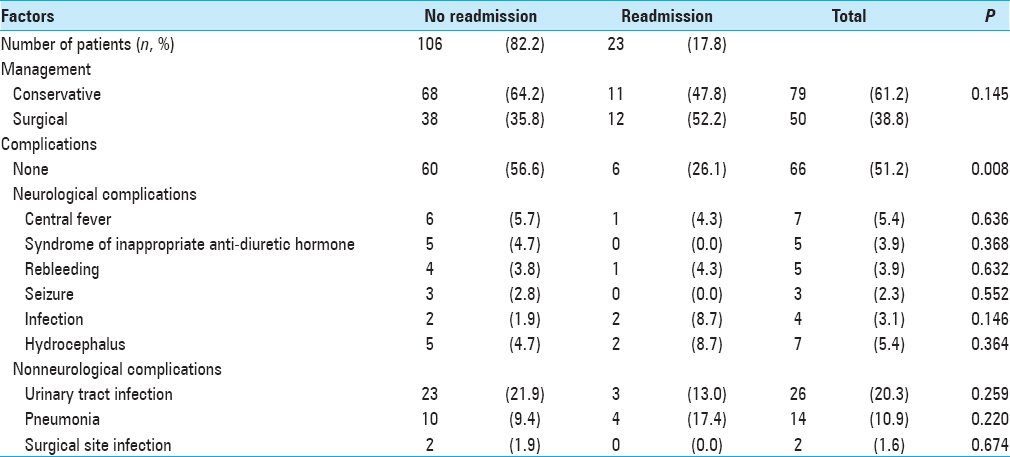
Patients with no complications were less likely to be readmitted (P = 0.008). There were no significant differences noted in treatment modalities compared with rates of readmission. Treatment that the patients underwent and the types of inpatient complications are detailed in
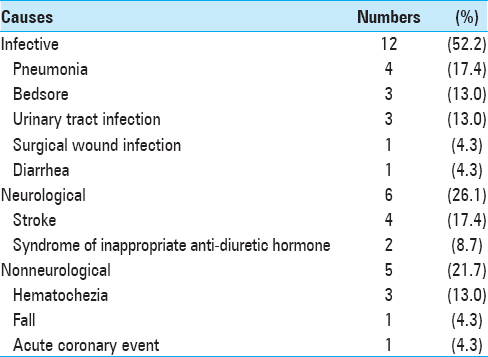
Of the 23 patients that were readmitted, approximately half were due to infective causes (n = 12, 52.5%) such as urinary tract infection, pneumonia, and surgical wound infections. The remaining etiologies for readmissions were divided into neurological etiologies (n = 6, 26.1%) and nonneurological etiologies (n = 5, 21.7%). The list of reasons for readmission is detailed in
DISCUSSION
Our study showed a statistically significant correlation between elevated pretransfer procalcitonin levels and incidence of readmission (P = 0.037). Other factors such as patient's age, premorbid status, neurological status on admission, and length of stay were not shown to be significantly associated with readmissions.
We propose that procalcitonin levels correlate directly and indirectly with infective complications, which make up the majority of readmissions (52.2%).
Serum procalcitonin has been shown to be a useful biomarker for early diagnosis of sepsis. We postulate that the direct link between procalcitonin and readmissions is due to it directly identifying patients harboring possible subclinical infections prior to transfer. These may manifest in the later phases of rehabilitation at the intermediate care facility, leading to readmissions.
High serum procalcitonin is predictive of a poor neurological outcome in postcardiac arrest patients who were not septic. This is possibly due to the inflammatory response to hypoxia suffered. Immobility is a major risk factor for infective complications such as pneumonia, bed sores, and urinary tract infections.[
It is, however, important to note that procalcitonin serves only as a screening blood test. We therefore recommend that patients with raised serum procalcitonin should be carefully assessed clinically for symptoms and signs of infection, followed by further investigations as appropriate. Serial serum procalcitonin should also be monitored to ensure a downward trend or the return to normalcy prior to transfer to an intermediate care facility.
We initially expected other factors to be associated with readmissions. These included age, premorbid ambulatory status, GCS on admission, and length of stay in the intermediate care facility. We suggest that the effects of these other factors may have been diluted due to the relatively long stay in both groups of patients, at an average of 32.9 and 48.4 days in the patients without readmission and patients with readmission, respectively. We postulate that beyond a certain length of stay, the infective risks among both patient groups have possibly equalized due to the prolonged exposure with nosocomial pathogens.
The limitation of this study lies mainly in its retrospective nature, and the relatively smaller sample size obtained from a single tertiary center. The results should be corroborated with large multi-center studies. Further prospective studies would also aid to confirm our findings.
CONCLUSIONS
Readmission of patients leads to inefficient healthcare spending. This study helps to provide insight on the possible predictive factors resulting in neurosurgical readmissions. By reducing the incidence of readmissions, we are better able to optimize the allocation of our limited healthcare resources, in a cost-effective manner. Despite its retrospective nature, the data of the study were obtained over a relatively long 2½-year period, and the results of this study add to our knowledge the role of serum procalcitonin in readmissions in neurosurgical patients.
Financial support and sponsorship
Department of Neurosurgery, National Neuroscience Institute, Singapore.
Conflicts of interest
There are no conflicts of interest.
References
1. Al-Nawas B, Krammer I, Shah PM. Procalcitonin in diagnosis of severe infections. Eur J Med Res. 1996. 1: 331-3
2. al-Nawas B, Shah PM. Procalcitonin in patients with and without immunosuppression and sepsis. Infection. 1996. 24: 434-6
3. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993. 341: 515-8
4. Bettencourt P, Azevedo A, Pimenta J, Friões F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004. 110: 2168-74
5. Brennan NLast accessed on 2015 Aug 16. Available from: http://www.brookings.edu/~/media/research/files/reports/2009/8/21%20bpc%20qualityreport/0821_bpc_qualityreport.
6. Brogan E, Langdon C, Brookes K, Budgeon C, Blacker D. Respiratory infections in acute stroke: Nasogastric tubes and immobility are stronger predictors than dysphagia. Dysphagia. 2014. 29: 340-5
7. Last accessed on 2015 Aug 16. Available from: http://www.cms.hhs.gov/MedicareMedicaidStatSupp/.
8. .editors. Systems of care for survivors of TBI. Textbook of Traumatic Brain Injury. American Psychiatric Pub; 2004. p. 559-69
9. Engel A, Steinbach G, Kern P, Kern WV. Diagnostic value of procalcitonin serum levels in neutropenic patients with fever: Comparison with interleukin-8. Scand J Infect Dis. 1999. 31: 185-9
10. Fries M, Kunz D, Gressner AM, Rossaint R, Kuhlen R. Procalcitonin serum levels after out-of-hospital cardiac arrest. Resuscitation. 2003. 59: 105-9
11. Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997. 24: 1240-2
12. Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Piña IL. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007. 167: 1998-2005
13. Gorzoni ML, Pires SL. Deaths in nursing homes. Rev Assoc Med Bras. 2011. 57: 327-31
14. Hayashida H, Kaneko T, Kasaoka S, Oshima C, Miyauchi T, Fujita M. Comparison of the predictability of neurological outcome by serum procalcitonin and glial fibrillary acidic protein in postcardiac-arrest patients. Neurocrit Care. 2010. 12: 252-7
15. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010. 303: 1716-22
16. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. N Engl J Med. 2009. 360: 1418-28
17. Kwaan MR, Vogler SA, Sun MY, Sirany AM, Melton GB, Madoff RD. Readmission after colorectal surgery is related to preoperative clinical conditions and major complications. Dis Colon Rectum. 2013. 56: 1087-92
18. Manière D. Complications of immobility and bed rest. Prevention and management. Rev Prat. 2012. 62: 1013-23
19. Martínez R, Gaul C, Buchfelder M, Erbguth F, Tschaikowsky K. Serum procalcitonin monitoring for differential diagnosis of ventriculitis in adult intensive care patients. Intensive Care Med. 2002. 28: 208-10
20. Masel BE, DeWitt DS. Traumatic brain injury: A disease process, not an event. J Neurotrauma. 2010. 27: 1529-40
21. Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998. 24: 680-4
22. Meisner M, Tschaikowsky K, Schmidt J, Schuttler J. Procalcitonin (PCT): A novel parameter for diagnosis and monitoring of bacterial inflammation and sepsis. Anaesth Intensive Med. 1996. 10: 529-39
23. Melis RJ, Olde Rikkert MG, Parker SG, van Eijken MI. What is intermediate care?. BMJ. 2004. 329: 360-1
24. Michtalik HJ, Yeh HC, Campbell CY, Haq N, Park H, Clarke W. Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. Am J Cardiol. 2011. 107: 1191-5
25. Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000. 26: 1193-200
26. Schwarz S, Bertram M, Schwab S, Andrassy K, Hacke W. Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med. 2000. 28: 1828-32
27. Titsworth WL, Hester J, Correia T, Reed R, Guin P, Archibald L. The effect of increased mobility on morbidity in the neurointensive care unit. J Neurosurg. 2012. 116: 1379-88
28. Tomio R, Akiyama T, Shibao S, Yoshida K. Procalcitonin as an early diagnostic marker for ventriculoperitoneal shunt infections. Surg Infect (Larchmt). 2013. 14: 433-6
29. Last accessed on 2004 May 06. Available from: http://www.nlm.nih.gov/mesh/meshhome.html.
30. Viallon A, Zeni F, Lambert C, Pozzetto B, Tardy B, Venet C. High sensitivity and specificity of serum procalcitonin levels in adults with bacterial meningitis. Clin Infect Dis. 1999. 28: 1313-6