- Department of Neurosurgery, Keio University School of Medicine, Shinjuku-Ku, Tokyo, 160-8582, Japan
- Division of Diagnostic Pathology, Keio University School of Medicine, Shinjuku-Ku, Tokyo, 160-8582, Japan
Correspondence Address:
Yuki Kuranari
Department of Neurosurgery, Keio University School of Medicine, Shinjuku-Ku, Tokyo, 160-8582, Japan
DOI:10.4103/sni.sni_231_18
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Yuki Kuranari, Ryota Tamura, Shuji Mikami, Kentaro Ohara, Masahiro Toda, Kazunari Yoshida. Severe headache in a patient with meningioma showing extensive dural tail correlates with IgG4-positive plasma cells and eosinophils: A case report and review of literature. 08-Oct-2018;9:202
How to cite this URL: Yuki Kuranari, Ryota Tamura, Shuji Mikami, Kentaro Ohara, Masahiro Toda, Kazunari Yoshida. Severe headache in a patient with meningioma showing extensive dural tail correlates with IgG4-positive plasma cells and eosinophils: A case report and review of literature. 08-Oct-2018;9:202. Available from: http://surgicalneurologyint.com/surgicalint-articles/9028/
Abstract
Background:Meningiomas originate from meningothelial cells of the arachnoid membrane. Few cases of meningioma with infiltration of inflammatory cells, such as lymphocytes and plasma cells, have been reported, and the mechanisms underlying meningioma-induced inflammatory reactions have not been fully elucidated.
Case Description:In this study, we report an extremely rare case of meningioma with infiltration of both IgG4-positive plasma cells and eosinophils showing extensive dural tail and reactive inflammation of the surrounding arachnoid tissue. The main clinical manifestation was a severe headache, which was improved by surgical excision of the tumor.
Conclusion:Only 8 cases of meningioma with IgG4-positive plasma cells have been reported, and only one case exhibited eosinophil infiltration. IgG4-related inflammatory response might mediate inflammation in surrounding tissue, resulting in thickening of the dura adjacent to a meningioma and severe headache. The mechanisms underlying inflammation by meningiomas require further investigation.
Keywords: Eosinophil, hypertrophic pachymeningitis, IgG4, meningioma
INTRODUCTION
Meningiomas originate from meningothelial cells of the arachnoid membrane.[
Sixty percent of the patients with meningiomas show dural thickening around the tumor, which manifests as the “dural tail sign.”[
This report describes an extremely rare case of a patient with severe headache caused by meningioma with extensive dural tail. Histopathological analysis of the excised tumor revealed IgG4-positive plasma cells and inflammatory eosinophil infiltration. We also review the relevant published literature on tumors including meningiomas with inflammatory cell infiltration.
CASE REPORT
Onset and course
A 49-year-old female presented with a 7 year history of severe headache. She was diagnosed with cluster headache at another hospital and began steroid therapy one-half year prior to presentation at our institution. Physical examination at the time of initial presentation revealed only severe headache without any neurological deficits. Laboratory examinations showed a C-reactive protein level of 0.07 mg/dl, white blood cell count of 6800/μl, erythrocyte sedimentation rate of 12 mm/hr, rheumatoid factor ≤5 IU/ml, ds DNA ≤1.2 IU/ml, PR3-ANCA ≤1.0 IU/ml and MPO-ANCA ≤1.0 IU/ml, SS-A ≤1.0 U/ml, SS-B ≤1.0 U/ml, and anti-cyclic citrullinated peptide antibody ≤0.5 U/ml. Serum IgG4 was within the normal range (10 mg/dl).
Preoperative radiographical findings
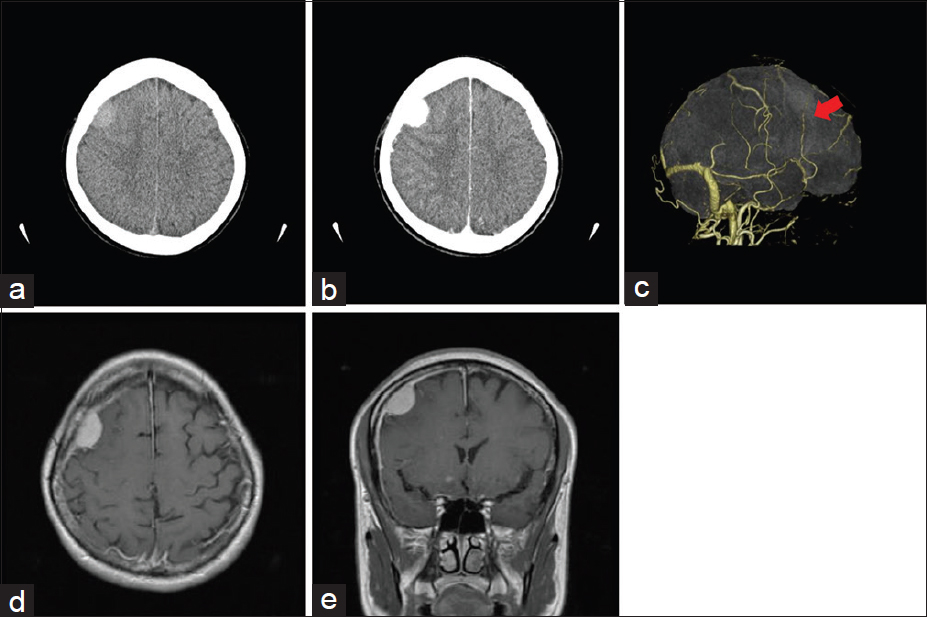
Head computed tomography showed a round lesion, 2.5 cm in diameter, originating primarily from the right frontal dura mater. The lesion showed isodensity without apparent calcification and hyperostosis [
Figure 1
Preoperative radiographic images. (a) Axial head computed tomography scan shows a round lesion, 2.5 cm in diameter, originating primarily from the right frontal dura mater. (b) Enhanced axial head computed tomography shows strong enhancement. (c) Head computed tomography angiography shows an apparent feeding artery (red arrow). (d) Contrast-enhanced axial magnetic resonance imaging shows a homogeneously enhanced extra-axial mass lesion. (e) Contrast-enhanced coronal magnetic resonance imaging shows enhanced extensive dural thickening around the tumor
Operation
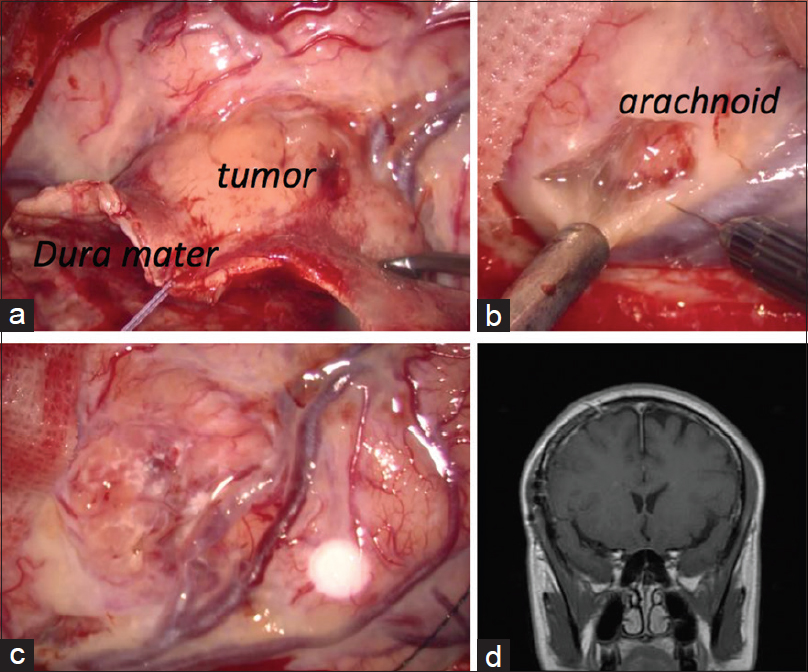
By frontal craniotomy, the feeding artery was identified on the attached dura mater and coagulated. The tumor was soft and grayish [
Figure 2
Operative findings. (a) The tumor was soft and grayish. Dura mater adjacent to the tumor was thickened. Capillary blood vessels on the inner layer of the dura mater were dilated. (b) Arachnoid around the tumor was yellowish. It was partially difficult to exfoliate the tumor from brain parenchyma. (c) Successful removal of the tumor was achieved by frontal craniotomy. Most of the thickened dura mater remained. (d) Contrast-enhanced coronal magnetic resonance imaging shows complete resection of the tumor. Most of the enhanced dura mater was still present
Histopathological findings
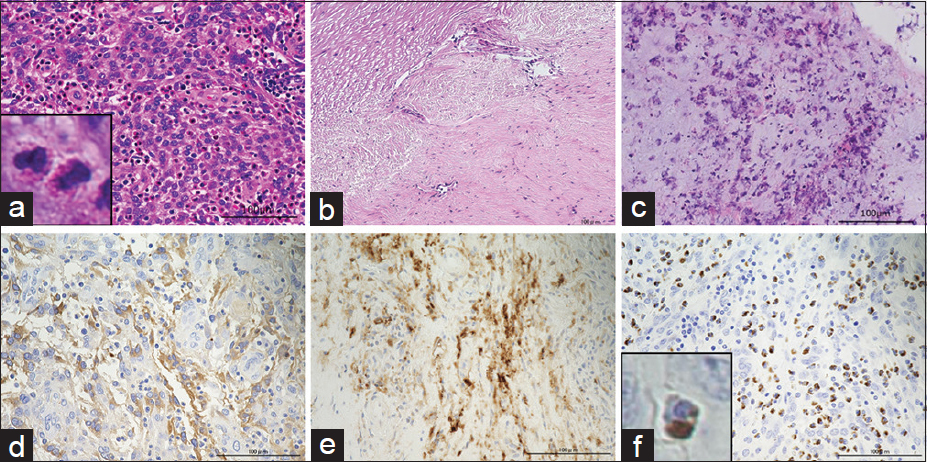
Histopathologically, the tumor was diagnosed as meningioma. In most parts of the tumor, oval-shaped arachnoid-like cells were proliferating like sheets [
Figure 3
Histopathological findings. (a-c) Hematoxylin-eosin staining, (d-f) immunohistochemical analysis of IgG, CD138, and IgG4. (a) In most parts of the tumor, oval-shaped arachnoid-like cells were proliferating like sheets. Numerous eosinophils were diffusely observed in the tumor. Square area demarcates eosinophils at higher magnification (×20, magnification bar: 100 μm). (b) Dura mater was strongly thickened and capillary blood vessels were dilated. Eosinophils were not observed in the dura mater. Degenerated collagenous fibers were observed (×20, magnification bar: 100 μm). (c) An inflammatory reaction was observed in the arachnoid attached to the tumor as evidenced by a small number of eosinophils and nuclear debris (×20, magnification bar: 100 μm). (d) Diffuse IgG-positive cell infiltration was observed in the tumor (×20, magnification bar: 100 μm). (e) CD138 (also known as syndecan-1, SDC1) is a highly specific marker for terminally differentiated normal plasma cells. Diffuse CD138-positive cells infiltration was also observed in the tumor (×20, magnification bar: 100 μm). (f) Most of the CD138-positive plasma cells also expressed IgG4. Diffuse IgG4-positive plasma cell infiltration was observed in the tumor. The ratio of IgG/IgG4 was 70%. Square area demarcates IgG4-positive plasma cells at high magnification (×20, magnification bar: 100 μm)
Postoperative course
Total resection of the tumor mass was confirmed by postoperative MRI [
DISCUSSION
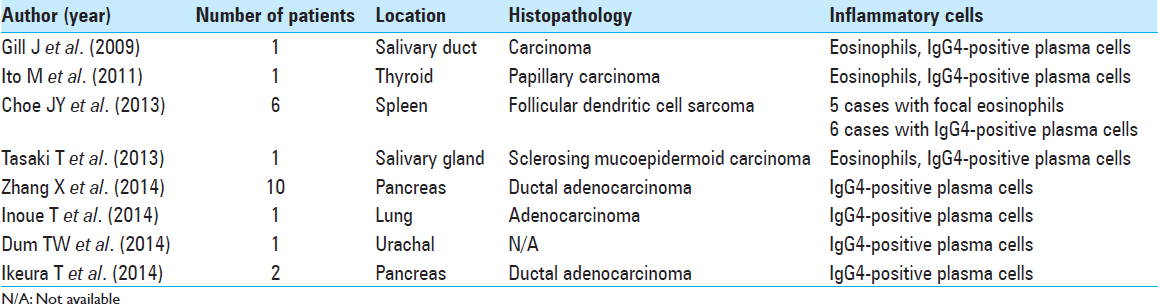
Infiltration of inflammatory cells has been known to occasionally occur in neoplasia. Twenty-three cases of neoplasia with intratumoral infiltration of IgG4-positive plasma cells and eosinophils have been previously reported [
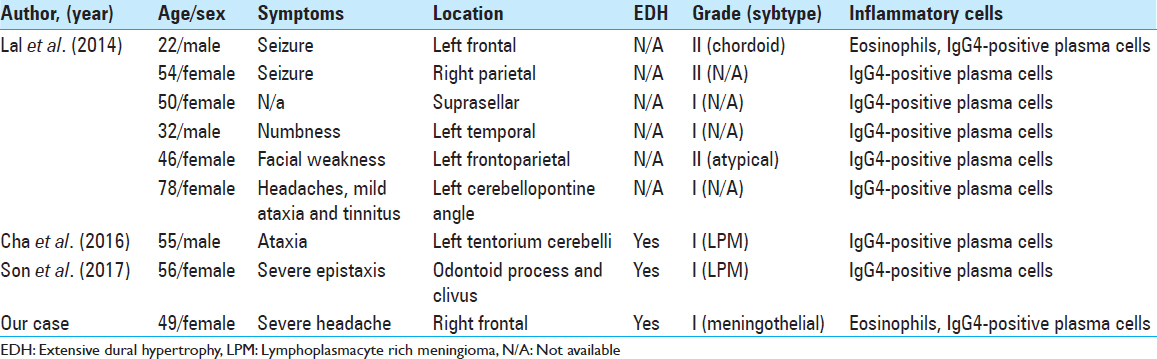
Infiltration of inflammatory cells in meningioma is extremely rare. Lal et al. summarized 16 cases of meningioma with infiltration of inflammatory cells.[
Tumor formation promoted by IgG4-mediated chronic inflammation should be considered in the present case. Previous reports demonstrated that pancreatic cancer was mediated by IgG4-related autoimmune pancreatitis.[
HP shows extensive thickened dura mater and sometimes forms pseudotumors mimicking meningioma.[
The histopathology of this tumor and dura mater is consistent with meningioma. The tumor was mainly formed by oval-shaped arachnoid-like cells, and dilated vessels were identified in the dura mater. As suggested in a previous report,[
In IgG4-related autoimmune reaction, interleukin-5/10/13 and transforming growth factor-β(TGF-β) produced by Th2 cells, regulatory T cells (Tregs), and mast cells are upregulated.[
The characteristics and pathophysiology of inflammation cell-rich meningioma are currently not fully elucidated because this type of meningioma was extremely rare. It might be related to some clinical information including radiographic images and patients’ symptoms. Further investigations were desirable to understand the mechanisms.
CONCLUSION
A meningioma exhibiting an extensive dural tail as well as IgG4-positive plasma cells and eosinophil infiltration has not been previously reported.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Baptista B, Casian A, Gunawardena H, D’Cruz D, Rice CM. Neurological manifestations of IgG4-related disease. Curr Treat Options Neurol. 2017. 19: 14-
2. Cha YJ, Lee SK, Chang JH, Kim SH. Report of a rare case of atypical lymphoplasmacyte-rich meningioma in the tentorium mimicking idiopathic hypertrophic pachymeningitis. Brain Tumor Pathol. 2016. 33: 216-21
3. Choe JY, Go H, Jeon YK, Yun JY, Kim YA, Kim HJ. Inflammatory pseudotumor-like follicular dendritic cell sarcoma of the spleen: A report of six cases with increased IgG4-positive plasma cells. Pathol Int. 2013. 63: 245-51
4. Dum TW, Zhang D, Lee EK. IgG4-related disease in a urachal tumor. Case Rep Urol. 2014. 2014: 275850-
5. Dziedzic T, Wojciechowski J, Nowak A, Marchel A. Hypertrophic pachymeningitis. Childs Nerv Syst. 2015. 31: 1025-31
6. Gill J, Angelo N, Yeong ML, McIvor N. Salivary duct carcinoma arising in igG4-related autoimmune disease of the parotid gland. Hum Pathol. 2009. 40: 881-6
7. Gleich GJ, Frigas E, Loegering DA, Wassom DL, Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979. 123: 2925-7
8. Gupta R, Khosroshahi A, Shinagare S, Fernandez C, Ferrone C, Lauwers GY. Does autoimmune pancreatitis increase the risk of pancreatic carcinoma? A retrospective analysis of pancreatic resections. Pancreas. 2013. 42: 506-10
9. Hirunwiwatkul P, Trobe JD, Blaivas M. Lymphoplasmacyte-rich meningioma mimicking idiopathic hypertrophic pachymeningitis. J Neuroophthalmol. 2007. 27: 91-4
10. Hosler MR, Turbin RE, Cho ES, Wolansky LJ, Frohman LP. Idiopathic hypertrophic pachymeningitis mimicking lymphoplasmacyte-rich meningioma. J Neuroophthalmol. 2007. 27: 95-8
11. Ikeura T, Miyoshi H, Uchida K, Fukui T, Shimatani M, Fukui Y. Relationship between autoimmune pancreatitis and pancreatic cancer: A single-center experience. Pancreatology. 2014. 14: 373-9
12. Inoue T, Hayama M, Kobayashi S, Oyaizu T, Nakazato Y, Honma K. Lung cancer complicated with IgG4-related disease of the lung. Ann Thorac Cardiovasc Surg. 2014. 20: 474-7
13. Ito M, Naruke Y, Mihara Y, So K, Miyashita T, Origuchi T. Thyroid papillary carcinoma with solid sclerosing change in IgG4-related sclerosing disease. Pathol Int. 2011. 61: 589-92
14. Jörg A, Henderson WR, Murphy RC, Klebanoff SJ. Leukotriene generation by eosinophils. J Exp Med. 1982. 155: 390-402
15. Lal A, Dahiya S, Gonzales M, Hiniker A, Prayson R, Kleinschmidt-DeMasters BK. IgG4 overexpression is rare in meningiomas with a prominent inflammatory component: A review of 16 cases. Brain Pathol. 2014. 24: 352-9
16. Larché M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003. 111: 450-63
17. Lu LX, Della-Torre E, Stone JH, Clark SW. IgG4-related hypertrophic pachymeningitis: Clinical features, diagnostic criteria, and treatment. JAMA Neurol. 2014. 71: 785-93
18. Mehta SH, Switzer JA, Biddinger P, Rojiani AM. IgG4-related leptomeningitis: A reversible cause of rapidly progressive cognitive decline. Neurology. 2014. 82: 540-2
19. Noshiro S, Wanibuchi M, Akiyama Y, Okawa S, Ohtaki S, Sugino T. IgG4-related disease initially presented as an orbital mass lesion mimicking optic nerve sheath meningioma. Brain Tumor Pathol. 2015. 32: 286-90
20. Qi ST, Liu Y, Pan J, Chotai S, Fang LX. A radiopathological classification of dural tail sign of meningiomas. J Neurosurg. 2012. 117: 645-53
21. Shibuya M. Pathology and molecular genetics of meningioma: Recent advances. Neurol Med Chir (Tokyo). 2015. 55: 14-27
22. Son HJ, Yu IK, Kim SM. Lymphoplasmacyte-rich meningioma with atypical angiomatous feature and an increased deposition of IgG4-positive plasma cells: An unusual case report. Int J Surg Pathol. 2018. 26: 93-7
23. Strehl JD, Hartmann A, Agaimy A. Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011. 64: 237-43
24. Takeuchi M, Sato Y, Ohno K, Tanaka S, Takata K, Gion Y. T helper 2 and regulatory T-cell cytokine production by mast cells: A key factor in the pathogenesis of IgG4-related disease. Mod Pathol. 2014. 27: 1126-36
25. Tasaki T, Matsuyama A, Tabata T, Suzuki H, Yamada S, Sasaguri Y. Sclerosing mucoepidermoid carcinoma with eosinophilia of the salivary gland: Case report and review of the literature. Pathol Int. 2013. 63: 125-31
26. Tien RD, Yang PJ, Chu PK. “Dural tail sign”: A specific MR sign for meningioma?. J Comput Assist Tomogr. 1991. 15: 64-6
27. Tsuboi H, Matsuo N, Iizuka M, Tsuzuki S, Kondo Y, Tanaka A. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther. 2012. 14: R171-
28. van der Vliet HJ, Perenboom RM. Multiple pseudotumors in IgG4-associated multifocal systemic fibrosis. Ann Intern Med. 2004. 141: 896-7
29. Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Analysis of the survival of mature human eosinophils: Interleukin-5 prevents apoptosis in mature human eosinophils. Blood. 1991. 78: 2542-7
30. Yamaguchi Y, Suda T, Suda J, Eguchi M, Miura Y, Harada N. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988. 167: 43-56
31. Yamashita H, Takahashi Y, Ishiura H, Kano T, Kaneko H, Mimori A. Hypertrophic pachymeningitis and tracheobronchial stenosis in IgG4-related disease: Case presentation and literature review. Intern Med. 2012. 51: 935-41
32. Zhang X, Liu X, Joseph L, Zhao L, Hart J, Xiao SY. Pancreatic ductal adenocarcinoma with autoimmune pancreatitis-like histologic and immunohistochemical features. Hum Pathol. 2014. 45: 621-7