- Department of Neurology, Kurashiki Central Hospital, Kurashiki, Japan.
- Department of Neurosurgery, Kurashiki Central Hospital, Kurashiki, Japan.
Correspondence Address:
Hiroyuki Ikeda, Department of Neurosurgery, Kurashiki Central Hospital, Kurashiki, Japan.
DOI:10.25259/SNI_727_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ryotaro Nukata1, Hiroyuki Ikeda2, Natsuki Akaike2, Yoshitaka Kurosaki2, Toshio Fujiwara2, Minami Uezato2, Masanori Kinosada2, Katsuro Shindo1, Masaki Chin2. Short-term aneurysm formation and rupture due to septic embolism diagnosed with a thrombus retrieved from another occluded artery. 14-Oct-2022;13:474
How to cite this URL: Ryotaro Nukata1, Hiroyuki Ikeda2, Natsuki Akaike2, Yoshitaka Kurosaki2, Toshio Fujiwara2, Minami Uezato2, Masanori Kinosada2, Katsuro Shindo1, Masaki Chin2. Short-term aneurysm formation and rupture due to septic embolism diagnosed with a thrombus retrieved from another occluded artery. 14-Oct-2022;13:474. Available from: https://surgicalneurologyint.com/surgicalint-articles/11924/
Abstract
Background: In rare cases, septic embolism is diagnosed on the basis of pathological findings of retrieved thrombi. Infected aneurysms can rapidly form and rupture after septic embolism, leading to a poor prognosis. We report a case of subcortical hemorrhage due to an infected aneurysm forming shortly after septic embolism in the left anterior cerebral artery.
Case Description: In this case, the diagnosis of septic embolism was made on the basis of pathological findings of a thrombus retrieved from the simultaneously occluded left middle cerebral artery, and endovascular embolization of the infected aneurysm was performed.
Conclusion: The pathological findings of a retrieved thrombus were useful for making a diagnosis of septic embolism. The possibility of short-term formation and rupture of an infected aneurysm after septic embolism should be noted. Endovascular embolization of occluded vessels due to septic embolism may prevent aneurysm formation and subsequent bleeding.
Keywords: Aneurysm, Pathology, Rupture, Septic embolism, Thrombus
INTRODUCTION
Recently, the usefulness of mechanical thrombectomy for large vessel occlusion due to infectious endocarditis has been reported.[
CASE REPORT
History and examination
A 58-year-old man collapsed on the street and was raced to our emergency room. He had a prior history of hypertension, dyslipidemia, and dysuria. Time last known well was 90 min before arrival. His blood pressure was 155/81 mmHg and his pulse rate was 90 bpm. His body temperature was 36.9°C. On neurologic examination, his Glasgow Coma Scale score was E3V1M5 (total score 9). He had total aphasia, right-sided hemiparesis, and left conjugate deviation of the eyes. His initial National Institutes of Health Stroke Scale (NIHSS) score was 20. Hematology test results were as follows: white blood cell count 9500/μL; C-reactive protein 3.46 mg/dL; brain natriuretic peptide <5.8 pg/mL; and D-dimer 1.1 μg/mL. Computed tomography (CT) of the head showed no intracranial hemorrhage and early ischemic change was absent (CT – Alberta Stroke Program Early CT Score 10). Diffusion-weighted images from magnetic resonance imaging of the head showed a high signal intensity area in the left MCA territory (diffusion-weighted image – Alberta Stroke Program Early CT Score 4), with a small high signal intensity area in the peripheral region of the left ACA [
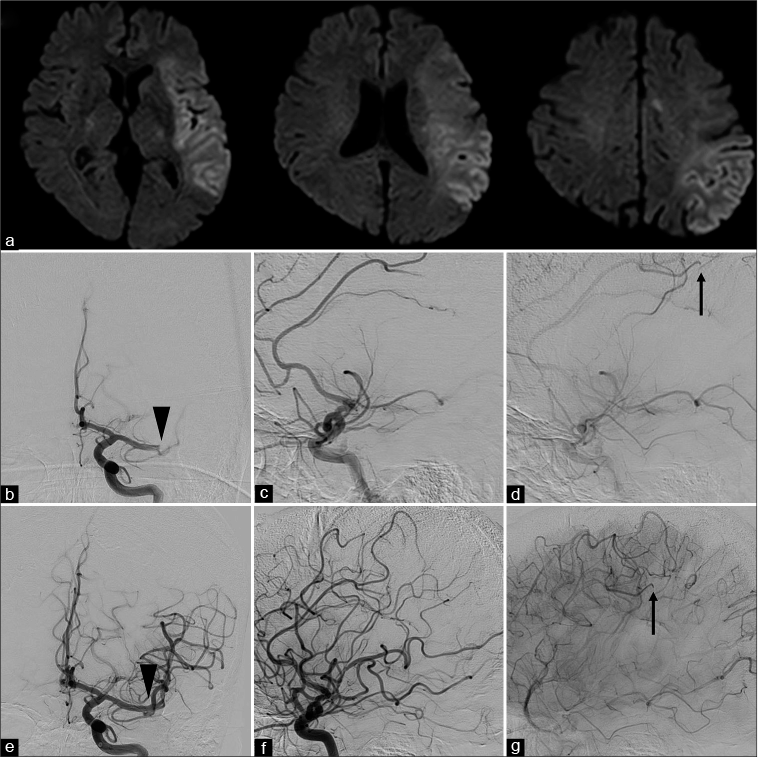
Figure 1:
Imaging findings before and during thrombus retrieval. (a) Magnetic resonance imaging diffusion-weighted imaging showing a high signal intensity area in the left middle cerebral artery territory (diffusion-weighted imaging – Alberta Stroke Program Early Computed Tomography Score 4), with a small high signal intensity area in the peripheral region of the left anterior cerebral artery. (b and c) Early-phase left internal carotid artery angiography before thrombectomy (b: anteroposterior view and c: lateral view) showing occlusion of the distal left middle cerebral artery (arrowhead). (d) Late-phase left internal carotid artery angiography before thrombectomy (lateral view) showing occlusion of the left peripheral pericallosal artery (arrow). (e and f) Early-phase left internal carotid artery angiography (e: anteroposterior view and f: lateral view) after thrombectomy showing recanalization of the M1 segment (arrowhead). (g) Late-phase left internal carotid artery angiography after thrombectomy showing occlusion of the left peripheral pericallosal artery (arrow).
Thrombectomy
The right femoral access was obtained. A 9-French balloon guiding catheter was placed in the left internal carotid artery. The left internal carotid artery angiography showed occlusion of the distal part of the M1 segment of the left MCA and the peripheral region of the pericallosal artery of the left ACA [
Course after thrombectomy
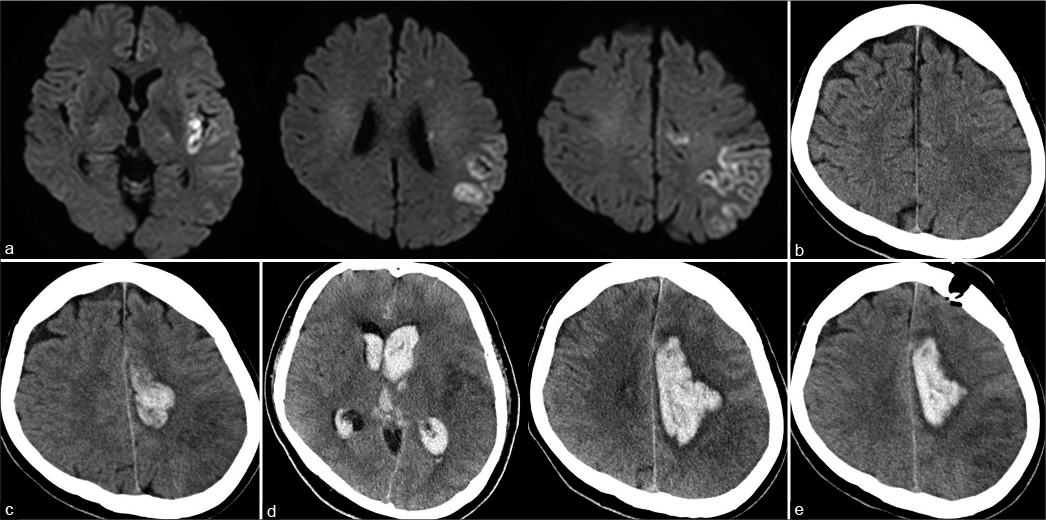
After thrombectomy, aphasia and right-side hemiparesis improved quickly. His NIHSS score improved to 4. Electrocardiographic monitoring showed no atrial fibrillation. Head magnetic resonance imaging obtained 4 h after thrombectomy showed a reduced diffusion-weighted imaging high signal intensity area in the left MCA territory with a small high signal intensity area in the peripheral region of the left ACA [
Figure 2:
Imaging findings after thrombus retrieval. (a) Head magnetic resonance imaging obtained 4 h after thrombectomy showing a reduced diffusion-weighted imaging high signal intensity area in the left middle cerebral artery territory with a small high signal intensity area in the peripheral region of the left anterior cerebral artery. (b) Head computed tomography obtained 24 h after thrombectomy showing a small high-density area around the infarct area in the anterior cerebral artery territory. (c) Head computed tomography obtained 38 h after thrombectomy showing subcortical hemorrhage in the left anterior cerebral artery territory and intraventricular hemorrhage. (d) Head computed tomography obtained 48 h after thrombectomy showing enlargement of subcortical hematoma and intraventricular distension. (e) Postoperative computed tomography showing residual hematoma around the infarct area in the left anterior cerebral artery.
The retrieved thrombus
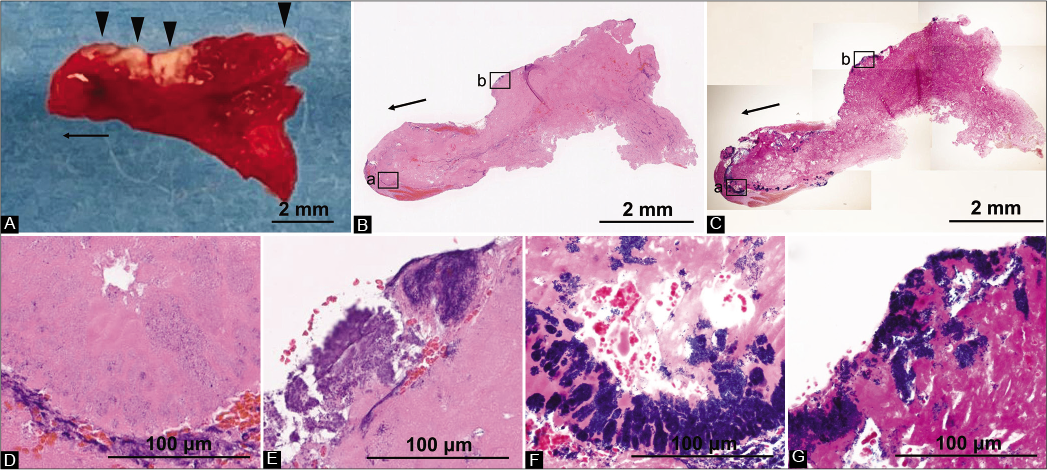
On the 6th day of hospitalization, the diagnosis was made on the basis of pathological findings of the thrombus retrieved from the left MCA. The thrombus was a single mass of 8 × 5 mm, with a visually recognizable red component mixed with a localized white component. It was firm and maintained the shape of the vessel at the distal part of the M1 segment and the bifurcation [
Figure 3:
Imaging findings of the thrombus. (A-C) Overall thrombus findings. The arrows show the proximal side of the M1 segment of the middle cerebral artery. (a) The thrombus was a single mass of 8 × 5 mm, with a visually recognizable red component mixed with a localized white component (arrowheads). It was firm and maintained the shape of the vessel at the distal part of the M1 segment and the bifurcation. (b) Hematoxylin-eosin staining showing that the thrombus consisted mainly of fibrin and platelets. (c) Consistent with the visually recognizable white component of the thrombus, Gram-positive cocci were observed. (D and E) D and E correspond to the squares “a” and square “b” in B, respectively. Hematoxylin-eosin staining showing the formation of spherical bacterial colonies at the margins of the thrombus and focally abundant neutrophils. (F and G) F and G correspond to the squares “a” and square “b” in C. The spherical bacteria at the margins of the thrombus were Gram staining positive.
Embolization
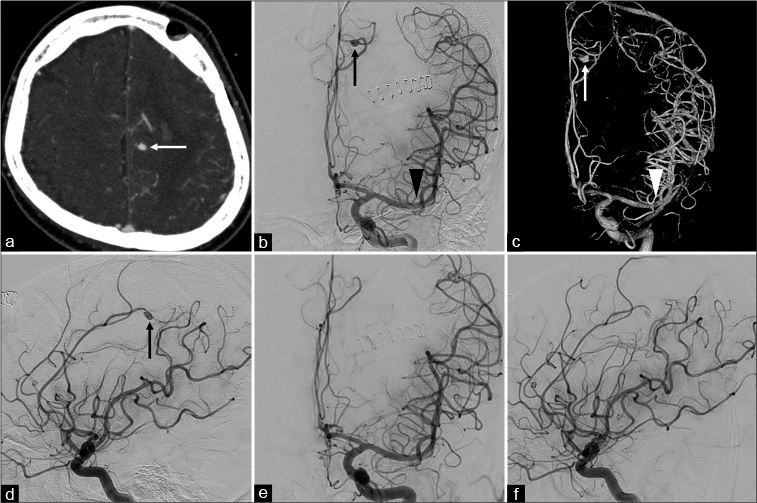
After the pathological diagnosis of the thrombus was made, an infected aneurysm was suspected of the source of the left frontal subcortical hemorrhage. CT angiography was performed, which revealed an aneurysm in the peripheral region of the left ACA [
Figure 4:
Imaging findings of the aneurysm before and during embolization. (a) An axial view of computed tomography angiography of the head showing an aneurysm in the peripheral left anterior cerebral artery (arrow). (b-d) The left internal carotid artery angiography (b: anteroposterior view, c: three-dimensional anteroposterior view, and d: lateral view) showing a 5.8 mm saccular aneurysm in the peripheral region of the pericallosal artery of the anterior cerebral artery (arrows) and no aneurysm formation in the recanalized distal part of the left M1 segment (arrowheads). (e and f) The left internal carotid artery angiography after embolization (e: anteroposterior view and f: lateral view) showing occlusion of the aneurysm.
Postoperative course
A diagnosis of septic embolism was made, and ceftriaxone and vancomycin were administered. Streptococcus gallolyticus developed from the blood culture taken on the 6th day of hospitalization. The antibiotics were switched to aminobenzylpenicillin, and the patient was treated for 5 weeks after the blood culture became negative.
Because of his poor general condition, transesophageal echocardiography was not performed. Postoperative magnetic resonance angiography and CT angiography of the head showed no new intracranial aneurysms. On the 49th day of hospitalization, he was transferred to a rehabilitation hospital. At the time of transfer, he was still suffering from aphasia and right hemiparesis (NIHSS score of 21), with a modified Rankin scale score of 5.
DISCUSSION
The patient had a left MCA occlusion and a peripheral left ACA occlusion due to septic embolism. After thrombectomy, successful reperfusion of the left MCA was achieved, and his neurological symptoms resolved markedly [
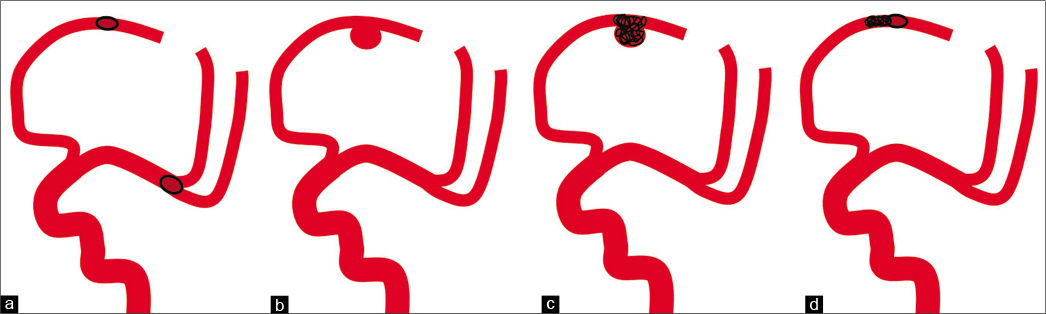
Figure 5:
Schema of this case. (a) Septic embolism was seen in the left middle cerebral artery and anterior cerebral artery. (b) An aneurysm was formed in the anterior cerebral artery with residual occlusion, but no aneurysm in the middle cerebral artery that was reperfused after thrombectomy. (c) The aneurysm in the peripheral anterior cerebral artery and the parent vessel was occluded. (d) Endovascular embolization of occluded vessels due to septic embolism may prevent aneurysm formation and subsequent bleeding.
White clots have been reported to be associated with atypical etiologies, in particular infective endocarditis.[
Infected aneurysm is caused by infection and destruction of the arterial wall. Infected emboli occlude the vessel and lead to cerebral infarction and subsequent focal infection. Infection can spread from the inside to the outside of the vessel wall, leading to aneurysm formation.[
This case met one major clinical criterion (positive blood culture) and two minor clinical criteria (fever and vascular lesions) of the revised Duke criteria, and the diagnosis of infected endocarditis was not definite.[
In this case, septic embolism was not recognized early because he had no fever on admission and blood tests showed only mildly elevated inflammatory markers. If pathological findings of the retrieved thrombus can be used to make a diagnosis of septic embolism, antimicrobial therapy and aneurysm evaluation can be initiated promptly. The problem is that it takes several days to make a pathological diagnosis, during which fatal outcomes due to hemorrhagic complications may occur. When recognizing an atypical thrombus on visual examination, it is important to keep in mind atypical etiologies such as septic embolism. If septic embolization results in residual peripheral occlusive lesions, close imaging follow-up should be performed with the possibility of rapid aneurysm formation and rupture in the future. In this case, if the occluded peripheral region of the left ACA had been found to be septic embolism early or before thrombectomy, embolization of this region might have prevented aneurysm formation and rupture [
CONCLUSION
The pathological findings of a retrieved thrombus were useful for making a diagnosis of septic embolism. The possibility of short-term formation and rupture of an infected aneurysm after septic embolism should be noted. Endovascular embolization of occluded vessels due to septic embolism may prevent aneurysm formation and subsequent bleeding.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alawieh A, Chaudry MI, Turner RD, Turk AS, Spiotta AM. Infectious intracranial aneurysms: A systematic review of epidemiology, management, and outcomes. J Neurointerv Surg. 2018. 10: 708-16
2. Hernández-Fernández F, Rojas-Bartolomé L, García-García J, Ayo-Martín Ó, Molina-Nuevo JD, Barbella-Aponte RA. Histopathological and bacteriological analysis of thrombus material extracted during mechanical thrombectomy in acute stroke patients. Cardiovasc Intervent Radiol. 2017. 40: 1851-60
3. Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm): Current options for diagnosis and management. Neurocrit Care. 2009. 11: 120-9
4. Kim JM, Jeon JS, Kim YW, Kang DH, Hwang YH, Kim YS. Forced arterial suction thrombectomy of septic embolic middle cerebral artery occlusion due to infective endocarditis: An illustrative case and review of the literature. Neurointervention. 2014. 9: 101-5
5. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000. 30: 633-8
6. Matsuzono K, Ishiyama Y, Higaki A, Namba K, Aoyama Y, Igarashi T. Successful endovascular coiling of infectious cerebral aneurysm following Staphylococcus haemolyticus endocarditis. J Int Med Res. 2021. 49: 3000605211058857
7. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018. 378: 11-21
8. Pasquereau-Kotula E, Martins M, Aymeric L, Dramsi S. Significance of Streptococcus gallolyticus subsp. Gallolyticus association with colorectal cancer. Front Microbiol. 2018. 9: 614
9. Ramos C, Mayo P, Trillo S, Gómez-Escalonilla C, Caniego JL, Moreu M. Management of large vessel occlusion stroke related to infective endocarditis: Is mechanical thrombectomy a safe option?. J Stroke Cerebrovasc Dis. 2020. 29: 105248
10. Sader E, Abdalkader M, Thom N, Nguyen TN, McDonald S, Greer D. Endovascular treatment of infective endocarditis-related acute large vessel occlusion stroke. J Stroke Cerebrovasc Dis. 2021. 30: 105775
11. Scharf EL, Chakraborty T, Rabinstein A, Miranpuri AS. Endovascular management of cerebral septic embolism: Three recent cases and review of the literature. J Neurointerv Surg. 2017. 9: 463-5
12. Settarzade E, Peker A, Topcuoglu MA, Arsava EM, Demircin M, Arat A. Clinical challenges associated with the endovascular treatment of acute stroke in a patient with infective endocarditis. J Cerebrovasc Endovasc Neurosurg. 2020. 22: 176-81
13. Sgreccia A, Duchmann Z, Desilles JP, Lapergue B, Labreuche J, Kyheng M. Association between acute ischemic stroke etiology and macroscopic aspect of retrieved clots: Is a clot’s color a warning light for underlying pathologies?. J Neurointerv Surg. 2019. 11: 1197-200
14. Thiene G, Basso C. Pathology and pathogenesis of infective endocarditis in native heart valves. Cardiovasc Pathol. 2006. 15: 256-63
15. Wang JL, Hinduja AP, Powers CJ. Successful coil embolization of a ruptured mycotic aneurysm that developed three days after septic embolic infarction: Case report and review of the literature. J Clin Neurosci. 2017. 39: 95-8