- Department of Neurosurgery, National Cerebral and Cardiovascular Center, Fujishiro-dai, Suita, Osaka, Japan
- Department of Radiology, National Cerebral and Cardiovascular Center, Fujishiro-dai, Suita, Osaka, Japan
- Department of Pathology, National Cerebral and Cardiovascular Center, Fujishiro-dai, Suita, Osaka, Japan
- Department of Center Research Institute, National Cerebral and Cardiovascular Center, Fujishiro-dai, Suita, Osaka, Japan
Correspondence Address:
Koji Iihara
Department of Center Research Institute, National Cerebral and Cardiovascular Center, Fujishiro-dai, Suita, Osaka, Japan
DOI:10.4103/2152-7806.198733
Copyright: © 2017 Surgical Neurology International This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Makoto Isozaki, Hiroharu Kataoka, Kazuhito Fukushima, Hatsue Ishibashi-Ueda, Naoaki Yamada, Hidehiro Iida, Koji Iihara. Silent ischemic lesion laterality in asymptomatic internal carotid artery stenosis relates to reduced cerebral vasoreactivity. 19-Jan-2017;8:6
How to cite this URL: Makoto Isozaki, Hiroharu Kataoka, Kazuhito Fukushima, Hatsue Ishibashi-Ueda, Naoaki Yamada, Hidehiro Iida, Koji Iihara. Silent ischemic lesion laterality in asymptomatic internal carotid artery stenosis relates to reduced cerebral vasoreactivity. 19-Jan-2017;8:6. Available from: http://surgicalneurologyint.com/surgicalint_articles/silent-ischemic-lesion-laterality-in-asymptomatic-internal-carotid-artery-stenosis-relates-to-reduced-cerebral-vasoreactivity/
Abstract
Background:We investigated the relationship between silent ischemic lesions, defined as hyperintense lesions on T2-weighted magnetic resonance imaging scans of brain white matter and cerebral hemodynamics (baseline cerebral blood flow and cerebral vasoreactivity).
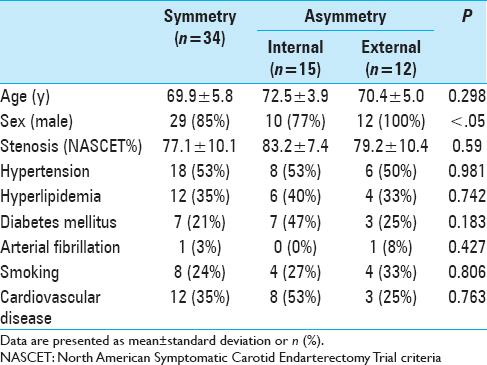
Methods:Between January 2007 and December 2012, 61 patients with asymptomatic internal carotid artery stenosis were evaluated for asymptomatic silent ischemic lesions, acute infarction, and cerebral hemodynamics. Patients were divided into 2 groups based on silent ischemic lesion distribution; the Symmetry group (n = 34) included patients who showed symmetrical distribution of lesions (or had no lesions), and the Asymmetry group (n = 27) included patients with a greater number of lesions in the ipsilateral than that in the contralateral hemisphere. The Asymmetry group was further divided into Internal (n = 15) and External (n = 12) types.
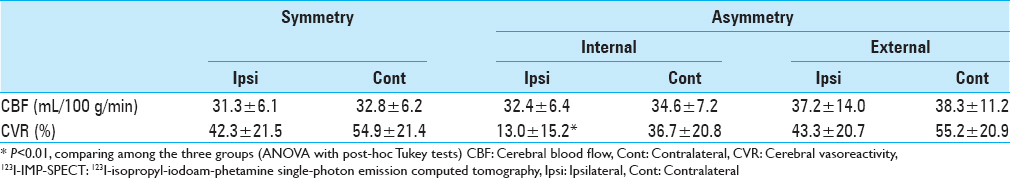
Results:Two External-type patients (17%) showed spotty asymptomatic acute infarction in the ipsilateral hemisphere. There were no significant differences in patient characteristics, histopathological findings, vascular risk factors, or cerebral blood flow values between the groups. The mean cerebral vasoreactivity value in the ipsilateral hemisphere for the Internal type was 13.0 ± 15.2% (range: −11.4% to 41.6%), which was significantly lower than values of the contralateral hemisphere (36.7 ± 20.8%; range: 3.9% to 75.7%; P <.01 and ipsilateral hemispheres of the other groups>P <.01>
Conclusions:The finding that increased ipsilateral asymmetrical silent ischemic lesions correlated with cerebral vasoreactivity reduction may help predict the risk of cerebral infarction in patients with asymptomatic internal carotid artery stenosis.
Keywords: Cerebral blood flow, cerebral vasoreactivity, internal carotid artery stenosis, silent ischemic lesions
INTRODUCTION
The therapeutic strategy for patients with asymptomatic carotid artery stenosis is highly controversial.[
MATERIALS AND METHODS
Participants
This study protocol was governed by the guidelines of the national government based on the Helsinki Declaration revised in 1983, and was approved by the Institutional Research and Ethics Committee of our hospital. Informed consent was obtained from all study participants in the study.
Between January 2007 and December 2012, 285 patients with internal carotid artery (ICA) stenosis were admitted to our hospital for CEA. Among them, 61 patients with unilateral asymptomatic ICA stenosis (51 men) with a mean age of 70 ± 5 years were included in this retrospective study. Patients with symptomatic ICA stenosis or with contralateral ICA stenosis (>50%) were excluded; all of the clinical data in this study were extracted from medical records. Symptomatic lesions were defined as lesions accompanied by a history of stroke, amaurosis fugax, or transient ischemic attacks involving the ipsilateral carotid territory occurring within 180 days of the initial assessment.[
Magnetic resonance imaging procedures
MR imaging was performed using a Magnetom Sonata 1.5T system (Siemens, Erlangen, Germany) with standard neck array and spine array coils. Plaque imaging was performed using magnetization-prepared rapid gradient-echo (MPRAGE) in transaxial sections using a null blood condition (effective inversion time, 660 ms; TR, 1500 ms) and the water excitation technique to suppress fat signals.[
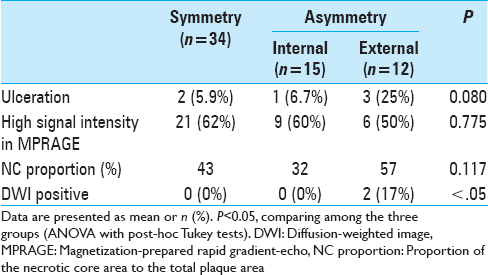
Two observers, a neurosurgeon and a radiologist, evaluated the signal intensity of the plaques on MPRAGE relative to the signal intensity in the adjacent muscle (typically the sternocleidomastoid muscle), as measured by placing a circular region of interest, 5–8 mm in diameter, on a standard console of the clinical MR system. If the plaque displayed a signal intensity of 200% or more of the muscle intensity in any area or section in the plaque, it was categorized as “high signal intensity.” Otherwise, the plaque was categorized as “low signal intensity.”[
Single-photon emission computed tomography procedures
Preoperative clinical single-photon emission computed tomography (SPECT) studies followed the dual table autoradiographic (DTARG) protocol, with dual administration of iodoamphetamine.[
Histopathological procedures
The CEA specimens were immediately fixed in HistoChoice® Tissue Fixative (Amresco, Inc., Solon, OH, USA) for 48 hours and decalcified with ethylenediaminetetraacetic acid. Subsequently, the specimens were divided into 5-mm blocks (starting at the CA bifurcation and extending rostrally along the ICA) and embedded in paraffin. From each 5-mm block, 3-µm sections were obtained and labeled with hematoxylin, eosin, and Masson's trichrome stain for histological evaluation. The necrotic core was defined as a core area of atheromatous plaques consisting of necrotic macrophages, cholesterol crystals, and (occasionally) hemorrhage. The proportion of the necrotic core area to the total plaque area (NC proportion) was measured using a computer-based morphometric system (WinRoof, Mitani Co., Ltd., Ishikawa, Japan). Each section was histopathologically evaluated by an experienced histopathologist.[
Statistical analysis
Differences in the bilateral hemodynamic parameters were statistically compared using repeated-measures analysis of variance (ANOVA) with post-hoc Tukey tests. Differences in the hemispheric values were compared using ANOVA and post-hoc Scheffe's F-test. Qualitative patient demographics were compared using the Chi-square test. A probability value of <.05 was considered a statistically significant difference.
RESULTS
The 61 study patients were divided into 2 groups based on the distribution of the SILs; the Symmetry group (n = 34), which included patients who showed symmetrical distribution of SILs or did not have any SILs, and the Asymmetry group (n = 27), which included patients with a greater number of SILs in the ipsilateral than in the contralateral hemisphere. The SILs of the patients in the Asymmetry group were further divided into 2 subtypes [
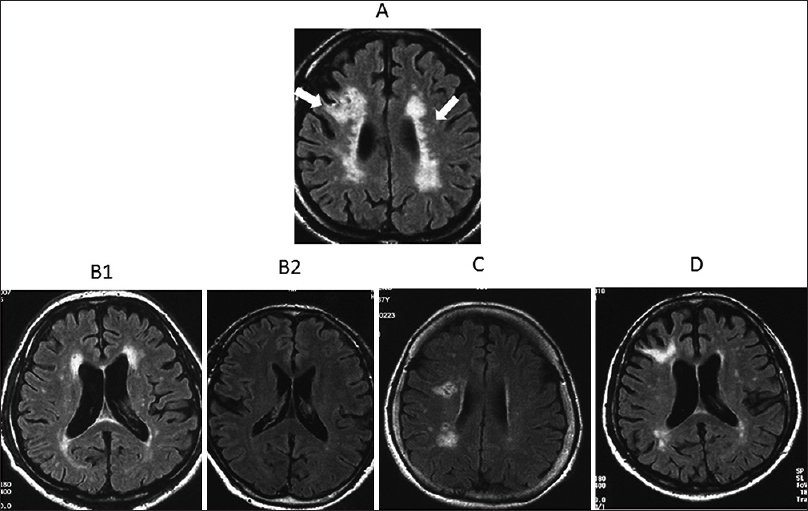
Figure 1
Patients were classified into groups on the basis of the distribution and location of silent ischemic lesions. (A) Silent ischemic lesions (SILs) were defined as asymptomatic hyperintense lesions on fluid-attenuated inversion recovery images in the white matter or as periventricular lesions (arrows). (B) Cases with symmetrical distribution of SILs (B1) or without SILs (B2) were categorized into the Symmetry group. (C) Cases with asymmetric SILs in the subcortical or deep white matter only were classified as the Asymmetry group (Internal type). (D) Cases with asymmetric SILs involving the cortex were classified as the Asymmetry group (External type)
The hemodynamic parameters measured with SPECT are presented in
DISCUSSION
Previous studies have shown that the extent of white matter lesions correlates with acute subcortical infarcts, which may be a risk factor for subsequent stroke.[
A significant association between the presence of white matter hyperintense lesions and the instability of carotid plaques as detected with MR plaque imaging, including the risk of intraplaque hemorrhage, has been reported in patients with symptomatic carotid artery disease.[
In this series, two cases of asymptomatic acute cerebral infarction detected with DWI were found to consist of multiple small lesions in patients with severe carotid artery stenosis. In a recent study that investigated the association between acute stroke patterns on DWI and carotid artery lesions, several disseminated small subcortical infarctions were recognized as a new stroke pattern, which were thought to be caused by multiple emboli or by the breakup of one large embolus.[
The main strength of our study was the comparison of FLAIR images as an advanced MR imaging tool, with quantitative CBF and CVR values measured using the DTARG protocol and 123I-Iodoamphetamine.[
CONCLUSION
In conclusion, we report that the presence of increased asymmetrical SILs in the deep white matter is associated with impaired CVR, which may indicate a poor collateral network in the ipsilateral hemisphere. These findings may help predict the risk of cerebral infarction in patients with asymptomatic ICA stenosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Altaf N, Morgan PS, Moody A, MacSweeney ST, Gladman JR, Auer DP. Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage. Radiology. 2008. 248: 202-9
2. Baird AE, Lövblad KO, Schlaug G, Edelman RR, Warach S. Multiple acute stroke syndrome: Marker of embolic disease?. Neurology. 2000. 54: 674-8
3. Bogousslavsky J, Regli F. Unilateral watershed cerebral infarcts. Neurology. 1986. 36: 373-7
4. Bogousslavsky J, Regli F. Borderzone infarctions distal to internal carotid artery occlusion: Prognostic implications. Ann Neurol. 1986. 20: 346-50
5. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998. 55: 1475-82
6. . Executive committee for the asymptomatic carotid atherosclerosis study. Endarterectomy for asymptomatic carotid stenosis. JAMA. 1995. 273: 1421-8
7. Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischemic stroke. J Neurol Neurosurg Psychiatry. 2005. 76: 793-6
8. Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: Randomised controlled trial. Lancet. 2004. 363: 1491-502
9. Hao H, Iihara K, Ishibashi-Ueda H, Saito F, Hirota S. Correlation of thin fibrous cap possessing adipophilin-positive macrophages and intraplaque hemorrhage with high clinical risk for carotid endarterectomy. J Neurosurg. 2011. 114: 1080-7
10. Helenius J, Soinne L, Salonen O, Kaste M, Tatlisumak T. Leukoaraiosis, ischemic stroke, and normal white matter on diffusion-weighted MRI. Stroke. 2002. 33: 45-50
11. Herskovits EH, Itoh R, Melhem ER. Accuracy for detection of simulated lesions: Comparison of fluid-attenuated inversion-recovery, proton density–weighted, and T2-weighted synthetic brain MR imaging. AJR Am J Roentgenol. 2001. 176: 1313-8
12. Hishikawa T, Iihara K, Yamada N, Ishibashi-Ueda H, Miyamoto S. Assessment of necrotic core with intraplaque hemorrhage in atherosclerotic carotid artery plaque by MR imaging with 3D gradient-echo sequence in patients with high-grade stenosis. Clinical article. J Neurosurg. 2010. 113: 890-6
13. Hobson RW, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993. 328: 221-7
14. Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nucl Med. 1994. 35: 2019-30
15. Isozaki M, Arai Y, Kudo T, Kiyono Y, Kobayashi M, Kubota T. Clinical implication and prognosis of normal baseline cerebral blood flow with impaired vascular reserve in patients with major cerebral artery occlusive disease. Ann Nucl Med. 2010. 24: 371-7
16. Kakkos SK, Sabetai M, Tegos T, Stevens J, Thomas D, Griffin M. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg. 2009. 49: 902-9
17. Kim KM, Watabe H, Hayashi T, Hayashida K, Katafuchi T, Enomoto N. Quantitative mapping of basal and vasareactive cerebral blood flow using, split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage. 2006. 33: 1126-35
18. Kuroda S, Kamiyama H, Abe H, Houkin K, Isobe M, Mitsumori K. Acetazolamide test in detecting reduced cerebral perfusion reserve and predicting long-term prognosis in patients with internal carotid artery occlusion. Neurosurgery. 1993. 32: 912-9
19. Kuroda S, Houkin K, Kamiyama H, Mitsumori K, Iwasaki Y, Abe H. Long-term prognosis of medically treated patients with internal carotid or middle cerebral artery occlusion: Can acetazolamide test predict it?. Stroke. 2001. 32: 2110-6
20. Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke. 2010. 41: e11-7
21. Mugler JP, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med. 1990. 15: 152-7
22. . North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325: 445-53
23. Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: A xenon-133 single-photon emission computed tomography study. Stroke. 2002. 33: 1857-62
24. Okazawa H, Tsuchida T, Kobayashi M, Arai Y, Pagani M, Isozaki M. Can the detection of misery perfusion in chronic cerebrovascular disease be based on reductions in baseline CBF and vasoreactivity?. Eur J Nucl Med Mol Imaging. 2007. 34: 121-9
25. Roh JK, Kang DW, Lee SH, Yoon BW, Chang KH. Significance of acute multiple brain infarction on diffusion-weighted imaging. Stroke. 2000. 31: 688-94
26. Szabo K, Kern R, Gass A, Hirsch J, Hennerici M. Acute stroke patterns in patients with internal carotid disease: A diffusion-weighted magnetic resonance imaging study. Stroke. 2001. 32: 1323-9
27. Timaran CH, McKinsey JF, Schneider PA, Littooy F. Reporting standards for carotid interventions from the Society for Vascular Surgery. J Vasc Surg. 2011. 53: 1679-95
28. Yamada N, Higashi M, Otsubo R, Sakuma T, Oyama N, Tanaka R. Association between signal hyperintensity on T1-weighted MR imaging of carotid plaques and ipsilateral ischemic events. Am J Neuroradiol. 2007. 28: 287-92