- Department of Endovascular Neurosurgery, Hospital Santa Teresa, Petropolis, Brazil.
- Department of Neurosurgery, Air Force Galeão Hospital, Rio de Janeiro, Brazil.
Correspondence Address:
Dan Zimelewicz Oberman, Department of Neurosurgery, Air Force Galeão Hospital, Rio de Janeiro, Brazil.
DOI:10.25259/SNI_97_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: José Alberto Almeida Filho1, Dan Zimelewicz Oberman2, Diogo Gonçalves Freitas1, Rodrigo Azeredo Costa1, Thiago Dantas S. Brandão1, Orlando Teixeira Maia Junior1. Silk + flow-diverter stent for the treatment of intracranial aneurysms associated with balloon angioplasty: A retrospective study. 05-May-2023;14:160
How to cite this URL: José Alberto Almeida Filho1, Dan Zimelewicz Oberman2, Diogo Gonçalves Freitas1, Rodrigo Azeredo Costa1, Thiago Dantas S. Brandão1, Orlando Teixeira Maia Junior1. Silk + flow-diverter stent for the treatment of intracranial aneurysms associated with balloon angioplasty: A retrospective study. 05-May-2023;14:160. Available from: https://surgicalneurologyint.com/?post_type=surgicalint_articles&p=12317
Abstract
Background: The silk + flow-diverter stent is increasingly used to treat complex intracranial aneurysms including wide-neck and fusiform aneurysms. Balloon angioplasty has been used to better appose the flow diverter (FD) to the vessel wall and, thus, improve aneurysm occlusion rates and decrease periprocedural complications. Sparse data are available concerning the results of this technique. We report our experience with silk + FD associated with balloon angioplasty for the treatment of intracranial aneurysms.
Methods: A retrospective study was conducted on all patients treated by the silk + FD. Clinical charts, procedural data, and angiographic results were reviewed and compared between those treated with balloon angioplasty. A multivariate analysis was conducted to identify predictors of complications, occlusion, and outcome.
Results: Between July 2014 and May 2016, we identified 209 patients with 223 intracranial aneurysms. There were 176 (84.2%) women and 33 (15.8%) men. The most common stent size used was 4.5 mm in 101 patients (46.1%), followed by 4 mm in 57 patients (26%). Univariate analysis observed that stent diameter was significantly related to aneurysm occlusion (P P = 0.0008). Patients who had angioplasty without the use of a balloon have a 13.69-times-higher risk of complications (OR = 13.69; P = 0.0003). Older age, larger aneurysms, and the use of more than 1 FD device were predictors of recanalization.
Conclusion: Endovascular treatment of intracranial aneurysms with the silk + FD associated with balloon angioplasty is a safe and effective therapeutic option. Balloon angioplasty in combination with FD lowers the risk of complications. Higher complication rates and worse outcomes are associated with older age and large aneurysms.
Keywords: Aneurysm, Balloon angioplasty, Complications, Flow diverter, Occlusion
INTRODUCTION
Over the past decade, flow diverter (FD) technology has emerged as a new generation of endoluminal implants for reconstructing the parent artery and treating brain aneurysms.[
They aim to redirect blood flow away from the aneurysm, causing stagnation, parent artery remodeling, resulting in gradual thrombosis, and complete occlusion of the aneurysm sac.[
Device malapposition, underexpansion, and proximal migration are technical difficulties associated with FDs that can lead to delayed ischemia episodes or potentially life-threatening aneurysm rupture.[
Several supplementary procedures, such as coiling or the implantation of additional intrasaccular or intraparental artery devices such as FDs or stents, have been described to improve the safety and efficacy of FDs.[
Therefore, the aim of our study was to present the safety and efficacy of balloon angioplasty as an adjunctive management option within a single type of FD (Silk+) for the treatment of intracranial aneurysms and discuss the results of this technique.
MATERIALS AND METHODS
Population
Our Institutional Ethics Committee authorized this retrospective study. Between July 2014 and May 2016, we identified all patients treated in our database with the Silk + stent, with and without balloon angioplasty (Copernic RC [BALT extrusion, Montmorency, France] compliant microcatheter) for 1 or multiple intracranial aneurysms. Individual treatment alternatives were assessed, and an agreement was reached among experienced endovascular neurosurgeons.
All patients underwent conventional angiography of both the internal carotid arteries (ICAs) and the vertebral arteries. Then, 3D-rotational angiography was performed to depict the aneurysm morphology. Adult patients (over the age of 18) who met the inclusion criteria were enrolled in the study. Any antiplatelet medication contraindications, pregnancy, breast-feeding, and aneurysms that could be treated with coils alone were all ruled out.
Variables evaluated
In the present study, the presence of balloon in angioplasty was considered, dichotomized, as the independent variable of interest, and the covariates selected as possible confounders were as follows: Gender, age (<60; ≥60), topography (in the ICA, in location other than the carotid artery), O’Kelly-Marotta (OKM) scale (A,B, C and D), silk + aneurysm (1; ≠ 1), circulation (Anterior; posterior), smoking, hypertension, rupture, associated coils (All with a yes or no response), aneurysm diameter (The maximal dimension of the aneurysm), stent diameter, and dome neck ratio (considered in the model as quantitative).
Endovascular procedure and follow-up
Antiplatelet therapy was administered to all patients 5–7 days before treatment. The antiplatelet reactivity test was not done, and the daily dose was set at 100 mg of aspirin plus 75 mg of clopidogrel. Following discharge, dual antiplatelet therapy was continued for at least 6 months after treatment. In the case of acute subarachnoid hemorrhage (SAH), the patients were perioperatively administered a dual antiplatelet combination of acetylsalicylic acid (300 mg) and clopidogrel (300 mg). After the procedure, the patient is maintained on aspirin (100 mg daily) for life and clopidogrel (75 mg daily) for approximately 6 months.
All of the patients with unruptured aneurysms were given local anesthetic and conscious sedation, unless the patient was previously under general anesthesia due to SAH. The femoral artery was the preferred artery for puncture. A 6F or 8F artery access was used for the procedures. Intravenous heparin was given once arterial access was achieved.
Clinical outcome evaluation
Clinical and neurological evaluations were done right after the procedure, at the hospital discharge, and 30 days, 180 days, and a year later. The modified Rankin scale (mRS)[
Angiography outcome evaluation
Angiographical proof of full aneurysm closure was the study’s endpoint. The OKM scale for flow diversion was used to do 6-month and 1-year angiographic follow-ups based on the degree of filling (A: total filling, B: subtotal filling, C: entry remnant, and D: no filling).[
Statistical methods
Logistic regression models were developed considering as dependent variables: The occurrence of complications in the procedure or the occurrence of occlusions; and demographic and clinical variables as explanatory variables. Analyses were performed at the procedure level and in this case that we have the presence of data considered clustered data since each procedure can be performed more than once on the same patient. The main feature of pooled data is that results from the same pool are likely to be positively correlated. Statistical methods should take this into account; therefore, logistic regression models were then fitted using generalized estimating equations (GEE) with a symmetric correlation matrix.
The analysis took place in two stages: bivariate and multiple, in both odds ratios (ORs) and their respective 95% confidence intervals (CIs) were calculated. Initially, simple logistic regression models were fitted for each covariate. Those in which P < 0.25 were included in the multiple logistic regression analysis.[
Multicollinearity between independent variables was assessed. It is considered as a limit for the presence of multicollinearity if the tolerance indicator assumes values >0.602.
The absence of symptoms assessed by the scale mRS and measured over a year of follow-up, among patients operated on with or without angiographic balloon, was tested using logistic regression models with GEE with covariance structure 1st-order autoregressive, adjusted for baseline measurements. Bonferroni correction was used to adjust for multiple comparisons.
It was considered significant P < 0.05. Analyses were conducted using the SAS 9.4 application.
RESULTS
Patient characteristics
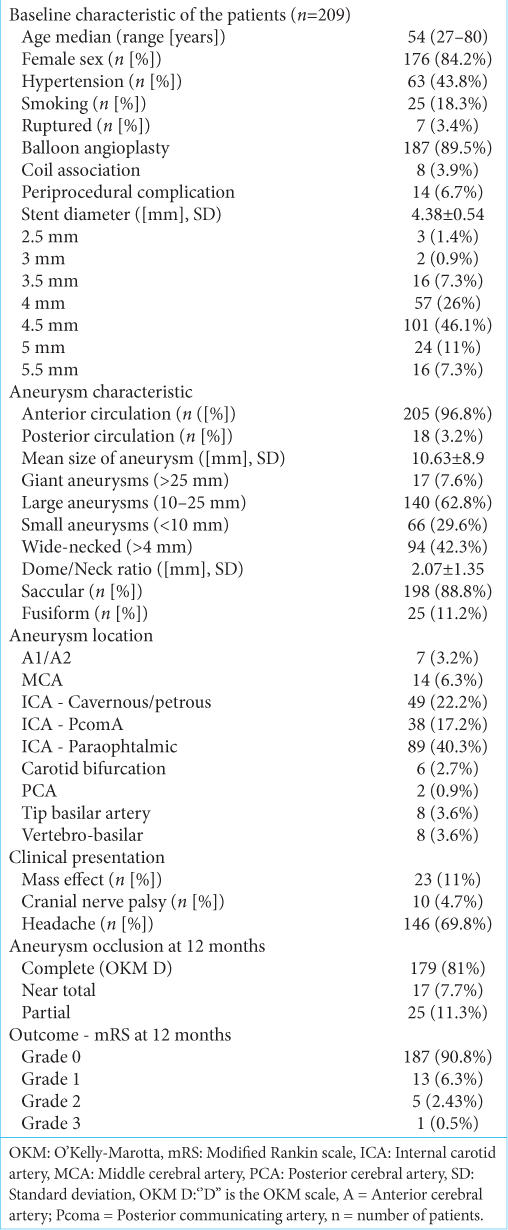
Demographic data, clinical presentation, aneurysm characteristics, location, size, and final outcome are summarized in
Two-hundred and nine patients with 223 intracranial aneurysms were identified. There were 176 (84.2%) women and 33 (15.8%) men, with a median age of 54 years (range, 27–80 years). Two-hundred and two (96.6%) patients were diagnosed with unruptured aneurysms, and 7 (3.4%) presented with ruptured aneurysms. Among the 202 patients with no previous hemorrhage, 46 (22.7%) were asymptomatic, whereas 146 (69.8%) complained of headache, 10 (4.7%) had cranial nerve palsy, and 23 (11%) had a mass effect.
Aneurysm characteristics
Aneurysms were located in the anterior circulation in 205 cases (96.8%) and in the posterior circulation in 18 cases (3.2%). The most common localization was the paraophthalmic ICA (89 [40.3%]), followed by the cavernous/petrous ICA (49 [22.2%]). Among the posterior circulation aneurysms, 2 (0.9%) were located at the posterior cerebral artery, 8 (3.6%) were at the tip of the basilar artery, and 8 (3.6%) were at the vertebrobasilar segment. One-hundred ninety eight aneurysms were saccular (89%) and 25 were fusiform (11%). Aneurysm median size was 10.63 ± 8.9 mm. The aneurysm dome/neck ratio ranged from 0.6 to 15 mm (average 2.1 mm). Wide-neck aneurysms (≥4 mm) were present in 94 aneurysms (42.3%).
Technical outcome
Most patients were treated with a single silk + stent with only 9 (4%) cases using two devices and a single case using 3 (1.35%) devices, on the same occasion. Aneurysm neck coverage was complete in almost all cases except in 3, where the device migrated. Balloon angioplasty was performed in 187 patients (89.5%).
Ten aneurysms (4.5%) were adjunctively coiled at the time of silk + placement. Stent diameter median size was 4.38 ± 0.54 mm. The most common stent size used was 4.5 mm in 101 patients (46.1%), followed by 4 mm in 57 patients (26%). Univariate analysis observed that stent diameter was significantly related to aneurysm occlusion (P < 0.05).
Complications in the procedure
Overall periprocedural complication rate was observed in 14 patients (6.7%). Among the 209 patients with follow-up, four patients presented periprocedural complication in the immediate period after the procedure, (two patients with mRS 0–4; one mRS 1–3; and one patient mRS 1–2). These periprocedural complications were attributed to ICA occlusion, transient ischemic attack, small frontal bleeding due to microguide aneurysm rupture, and silk+ stent twist, which may not be related with balloon angioplasty. All these patients recovered to their basal mRS at the end of the 1-year period follow-up. One patient presented delayed stent occlusion, 4 days after treatment. Final clinical outcomes in 209 patients included 188 with mRS = 0; 14 with mRS = 1; six patients with mRS = 2, and one patient with mRS = 3.
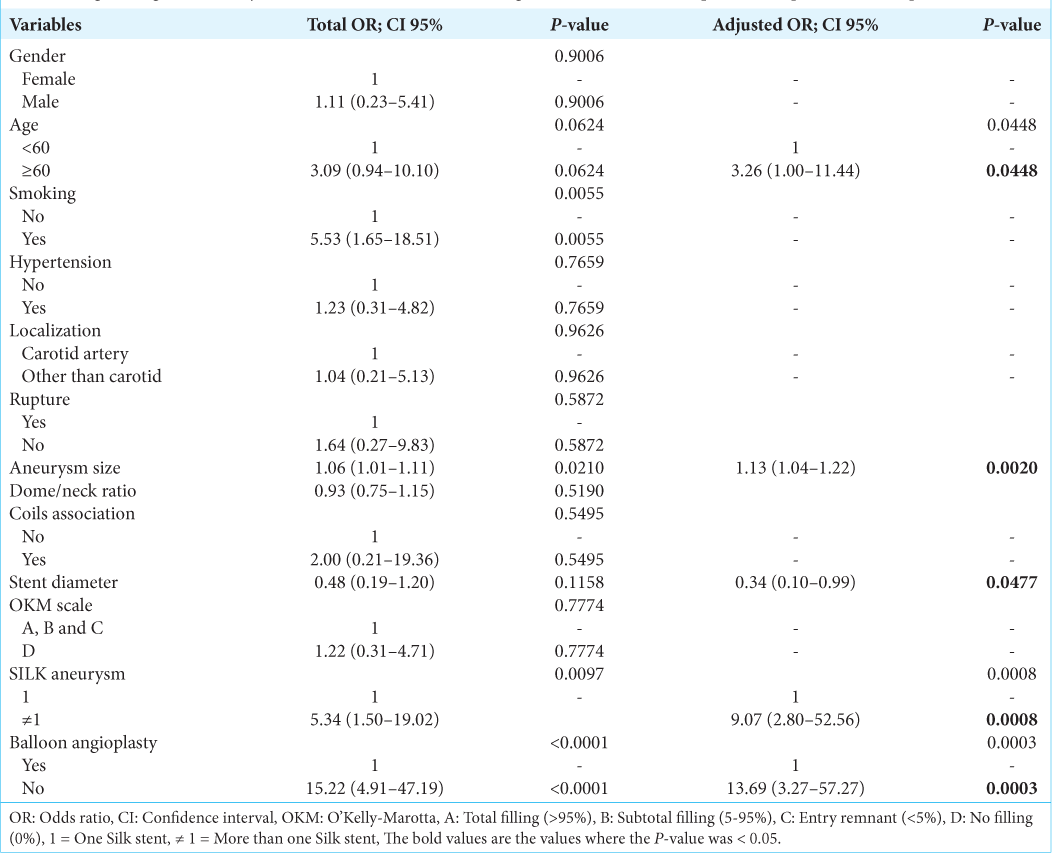
The bivariate analysis identified that the variables age, smoking, aneurysm diameter, stent diameter, and silk + aneurysm were associated with complications in the procedure and because they had P < 0.25, they were included in the multivariate analysis [
Occlusions in the procedure
Angiographic controls showed 179 complete occlusions (81%), 17 partial occlusion (7.7%), and 25 incomplete occlusions (11.3%) at 12 months. There were no procedural deaths reported in the acute and delayed period.
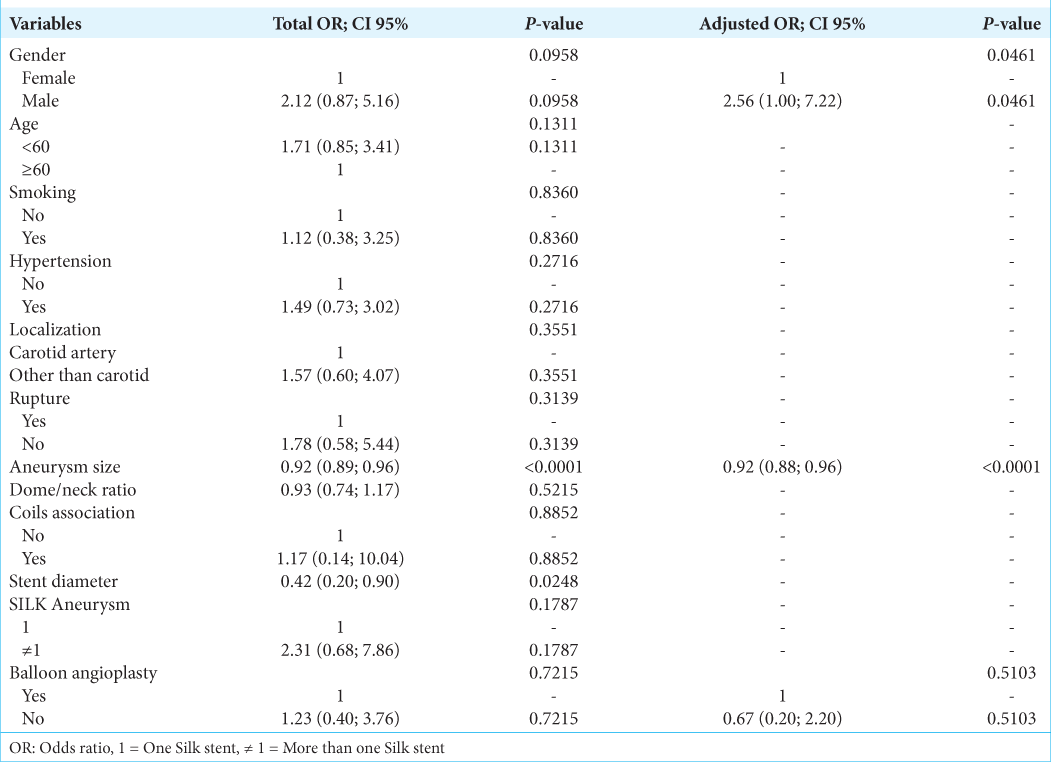
The bivariate analysis identified that the variables sex, age, aneurysm diameter, stent diameter, and silk + aneurysms were associated with occlusion in the procedure and because they had P < 0.25, they were included in the multivariate analysis [
The tolerance indicator for multicollinearity ranged from 0.92 to 0.96, indicating that there is no multicollinearity between the independent variables in the adjusted models.
Clinical outcome after balloon angioplasty
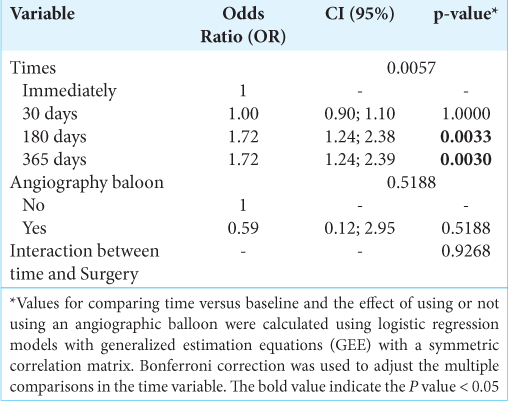
Initially, a model was adjusted in which the interaction between follow-up time and the use or not of a balloon angioplasty was adjusted, to verify whether the behavior of patients, during the follow-up period, regarding non-disability or dependence in activities daily differs between those who were operated with or without angiographic balloon [
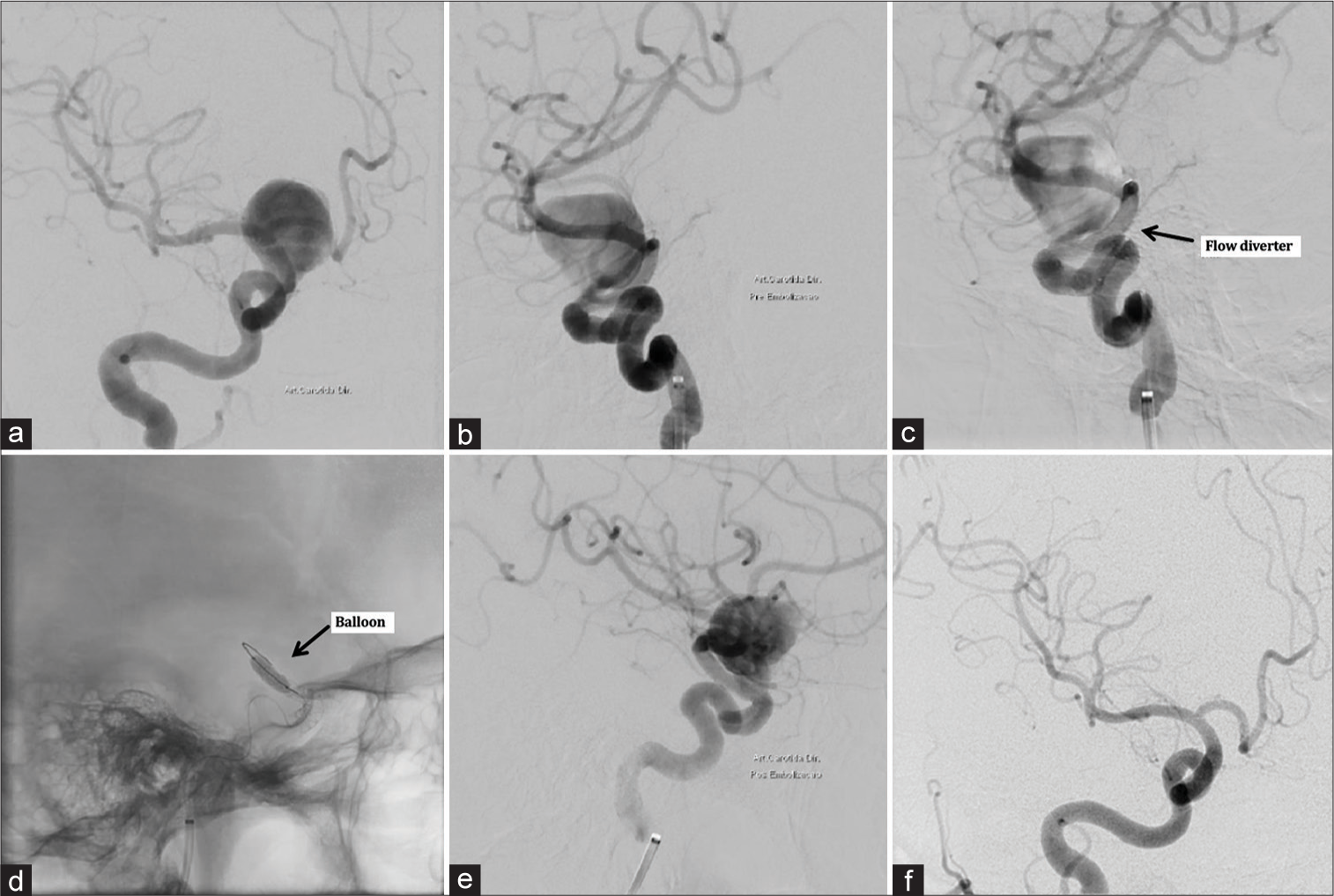
Figure 1:
Examples of pretreatment and 1-year posttreatment angiographic images of giant carotid-ophthalmic aneurysm treated using silk + flow diverter, showing aneurysm occlusion. (a) Pre-procedural conventional angiography shows a complex wide-neck giant carotid-ophthalmic aneurysm. (b) Lateral view. (c) The intraoperative views show the delivery of the silk + flow diverter (arrow). (d) Angiography showing balloon dilatation placement (arrow). (e) Immediate post-procedure angiography. (f) Angiographic control at 1-year demonstrating the reconstruction of the parent artery and total occlusion of the aneurysm.
Subsequently, a model was adjusted without the presence of an interaction between follow-up time and the use or not of a balloon angioplasty, and it was observed that only the effect of time was significant (P = 0.0057). Patients after 30 days of follow-up showed no significant difference in terms of the absence of symptoms when compared to the moment immediately after surgery (P = 1.0000). Patients after 180 days and 365 days of follow-up had a 72% greater chance of not having symptoms when compared to the moment immediately after surgery (P = 0.0033 and 0.0030, respectively). The effect of using or not using balloon angioplasty was not significantly different regarding the absence of symptoms (P = 0.5188).
DISCUSSION
This study demonstrates that endovascular treatment of cerebral aneurysms with the silk + stent in combination with balloon angioplasty is a safe and effective treatment option with minimal periprocedural problems. Furthermore, using balloon angioplasty throughout the procedure reduced the number of periprocedural complications. This work shows good technical success as well as immediate-term, short-term, and mid-term outcomes. These findings imply that placing a balloon angioplasty within FDs protects against periprocedural complications and is linked to a high rate of aneurysm occlusion.
The development of FDs for the thrombosis of wide-neck, fusiform, and giant aneurysms has elicited great enthusiasm in the neurointerventional community. Since the introduction in clinical practice, FDs devices have had excellent results.[
However, reports about the occurrence of procedure-related late thrombosis, fatal hemorrhage, and delayed complications have also been published.[
Incomplete vessel-wall apposition of FDs may also impede its ability to redirect blood flow away from the aneurysm sac, which then could interfere with stable intra-aneurysmal thrombus formation and flow stagnation, and may results in incomplete aneurysm occlusion, increase in periprocedural complications and delayed thromboembolic events.[
The process of establishing an endothelium, which consists of an intact layer of vascular endothelial cells over the FD, is known as endothelialization. This layer helps the surrounding vascular tone by supplying nitric oxide and prostaglandins, as well as acting as a physical barrier, potentially minimizing the risk of thrombosis and delayed cerebral ischemia,[
Several clinical researches, especially in coronary literature, highlight the potential for in-stent thrombosis and delayed ischemic events in incomplete stent apposition.[
For the treatment of cerebral aneurysms and the prevention of delayed ischemic consequences, accurate measurement of FDs deployment and wall apposition is critical.[
Recently, novel self-expandable devices have been introduced to reduce thromboembolic complications, facilitate navigability, improve radial force, and increase aneurysm occlusion rate.[
Balloon angioplasty has been used to fully open the FD or to attach its proximal segment in the parent artery wall, with excellent results.[
Although the silk + stent is self-expandable, the open cell design makes it exceedingly flexible, and it cannot ensure that all of the cells will line properly with the artery’s axis. Due to misalignment, cells at the distal and proximal borders may not entirely attach to the arterial wall. Self-expandable stents combined with balloon angioplasty could help enhance wall apposition.
In such circumstances, a second FD or stent is certainly an option, but it is not always feasible, particularly in a perforator-rich zone where the increased metal-to-artery ratio may result in perforator occlusion. In addition, our research found an increase in the number of complications, which is consistent with the findings of other researchers. Lubicz et al.[
With the expanding use of FDs to treat cerebral aneurysms, our standard recommendation of balloon angioplasty may be effective in enhancing wall apposition. Although we saw a decrease in the number of complications, this technique could lead to dissection or rupture of the parent artery due to balloon overinflation, as well as increased procedure duration and thromboembolic consequences.[
We believe that the association between silk+ and balloon angioplasty reduced periprocedural complications, as demonstrated in our cohort, which is best explained by the increased surface area of metal coverage provided by balloon angioplasty and increased wall apposition with the device. This increase in metal coverage is a consequence of best wall apposition due to the dilation of the balloon.
We also noted that intraprocedural age >60 years old, large aneurysm size, and use of more than 1 device are associated in the multivariate analysis with procedural complications, which are in accordance with the previous publications.[
Our research has some limitations. Because it was a retrospective study, it comes with its own set of limitations. We did not assess the antiplatelet response. Most likely, we have underestimated the percentage of subjects resistant to antiplatelet medication. Imaging follow-up was incompletely homogeneous and did not include postoperative imaging evaluation, other than DSA. Another limitation is that our study included small control groups (without balloon angioplasty). The majority of aneurysms were found in the ICA, which implies a lower risk of complications than those found elsewhere in the anterior circulation. Despite these limitations, this study answers various concerns that have previously gone unanswered, and it provides several independent predictors of problems, occlusion, and outcome.
CONCLUSION
Our research showed that balloon angioplasty inside an FD is both safe and effective. Our findings were based on a study of aneurysms treated with a silk + FD to promote wall apposition. Balloon angioplasty in combination with FD lowers the risk of complications. Complications are more likely in older patients, those with larger aneurysms, and those who utilize more than one FD device.
Our findings must be confirmed in larger cohorts, particularly from multicenter registries, and cannot be applied to all neurointerventional techniques. However, in certain instances, surgeons may consider placing a balloon angioplasty within a newly placed FD, which may help with occlusion rates and drastically reduce the procedure’s risk.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: Systematic review and meta-analysis. Neurosurgery. 2013. 73: 193-9
2. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: Five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. 2017. 80: 40-8
3. Berge J, Biondi A, Machi P, Brunel H, Pierot L, Gabrillargues J. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol. 2012. 33: 1150-5
4. Bhogal P, AlMatter M, Hellstern V, Ganslandt O, Bäzner H, Henkes H. The combined use of intraluminal and intrasaccular flow diversion for the treatment of intracranial aneurysms: Report of 25 cases. Neurointervention. 2018. 13: 20-31
5. Bonita R, Beaglehole R. Recovery of motor function after stroke. Stroke. 1988. 19: 1497-500
6. Byrne JV, Beltechi R, Yarnold JA, Birks J, Kamran M. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: A multicentre prospective study. PLoS One. 2010. 5: e12492
7. Cagnazzo F, Mantilla D, Lefevre PH, Dargazanli C, Gascou G, Costalat V. Treatment of middle cerebral artery aneurysms with flow-diverter stents: A systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017. 38: 2289-94
8. Carneiro A, Rane N, Küker W, Cellerini M, Corkill R, Byrne JV. Volume changes of extremely large and giant intracranial aneurysms after treatment with flow diverter stents. Neuroradiology. 2014. 56: 51-8
9. Chalouhi N, Satti SR, Tjoumakaris S, Dumont AS, Gonzalez LF, Rosenwasser R. Delayed migration of a pipeline embolization device. Neurosurgery. 2013. 72: ons229-34 discussion ons234
10. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G. Spontaneous delayed migration/shortening of the pipeline embolization device: Report of 5 cases. AJNR Am J Neuroradiol. 2013. 34: 2326-30
11. Clarençon F, Di Maria F, Gabrieli J, Shotar E, Degos V, Nouet A. Clinical impact of flat panel volume CT angiography in evaluating the accurate intraoperative deployment of flow-diverter stents. AJNR Am J Neuroradiol. 2017. 38: 1966-72
12. Cohen JE, Gomori JM, Moscovici S, Leker RR, Itshayek E. Delayed complications after flow-diverter stenting: Reactive in-stent stenosis and creeping stents. J Clin Neurosci. 2014. 21: 1116-22
13. Ding D, Starke RM, Durst CR, Gaughen JR, Evans AJ, Jensen ME. DynaCT imaging for intraprocedural evaluation of flow-diverting stent apposition during endovascular treatment of intracranial aneurysms. J Clin Neurosci. 2014. 21: 1981-3
14. Ding D, Starke RM, Evans AJ, Jensen ME, Liu KC. Balloon anchor technique for pipeline embolization device deployment across the neck of a giant intracranial aneurysm. J Cerebrovasc Endovasc Neurosurg. 2014. 16: 125-30
15. Douglas G, Van Kampen E, Hale AB, McNeill E, Patel J, Crabtree MJ. Endothelial cell repopulation after stenting determines in-stent neointima formation: Effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J. 2013. 34: 3378-88
16. Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct: Case report. Neurosurgery. 2010. 67: E313-4
17. Foin N, Gutiérrez-Chico JL, Nakatani S, Torii R, Bourantas CV, Sen S. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: Implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 2014. 7: 180-9
18. Gatto P, Dumonteil N, Boudou N, Van Rothem J, Lhermusier T, Bartorelli A. Incomplete stent apposition and very late stent thrombosis after everolimus eluting stent implantation and dual antiplatelet therapy interruption. A case of OCT guided therapy. Int J Cardiol. 2015. 180: 52-4
19. Cognard C, Pierot L, Anxionnat R, Ricolfi F. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: Clinical results of the Clarity GDC study. Neurosurgery. 2011. 69: 837-41
20. Heller R, Calnan DR, Lanfranchi M, Madan N, Malek AM. Incomplete stent apposition in Enterprise stent-mediated coiling of aneurysms: Persistence over time and risk of delayed ischemic events. J Neurosurg. 2013. 118: 1014-22
21. Hosmer DW, Lemeshow S, Sturdivant RX, editors. Applied Logistic Regression. New York: John Wiley and Sons; 2013. p.
22. Huang QH, Yang PF, Zhang X, Shi Y, Shao XM, Liu JM. Effects of flow diverter with low porosity on cerebral aneurysms: A numerical stimulative study. Zhonghua Yi Xue Za Zhi. 2010. 90: 1024-7
23. Ionita CN, Natarajan SK, Wang W, Hopkins LN, Levy EI, Siddiqui AH. Evaluation of a second-generation self-expanding variable-porosity flow diverter in a rabbit elastase aneurysm model. AJNR Am J Neuroradiol. 2011. 32: 1399-407
24. Jou LD, Mitchell BD, Shaltoni HM, Mawad ME. Effect of structural remodeling (retraction and recoil) of the pipeline embolization device on aneurysm occlusion rate. AJNR Am J Neuroradiol. 2014. 35: 1772-8
25. Kadirvel R, Ding YH, Dai D, Rezek I, Lewis DA, Kallmes DF. Cellular mechanisms of aneurysm occlusion after treatment with a flow diverter. Radiology. 2014. 270: 394-9
26. Kallmes DF, Brinjikji W, Cekirge S, Fiorella D, Hanel RA, Jabbour P. Safety and efficacy of the Pipeline embolization device for treatment of intracranial aneurysms: A pooled analysis of 3 large studies. J Neurosurg. 2017. 127: 775-80
27. Kato N, Yuki I, Ishibashi T, Ikemura A, Kan I, Nishimura K. Visualization of stent apposition after stent-assisted coiling of intracranial aneurysms using high resolution 3D fusion images acquired by C-arm CT. J Neurointerv Surg. 2020. 12: 192-6
28. Klisch J, Turk A, Turner R, Woo HH, Fiorella D. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. AJNR Am J Neuroradiol. 2011. 32: 627-32
29. Kühn AL, Rodrigues KM, Wakhloo AK, Puri AS. Endovascular techniques for achievement of better flow diverter wall apposition. Interv Neuroradiol. 2019. 25: 344-7
30. Kuriyama T, Sakai N, Beppu M, Sakai C, Imamura H, Masago K. Quantitative analysis of conebeam CT for delineating stents in stent-assisted coil embolization. AJNR Am J Neuroradiol. 2018. 39: 488-93
31. Lescher S, du Mesnil de Rochemont R, Berkefeld J. Woven Endobridge (WEB) device for endovascular treatment of complex unruptured aneurysms-a single center experience. Neuroradiology. 2016. 58: 383-90
32. Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: A prospective study in 29 patients with 34 aneurysms. Stroke. 2010. 41: 2247-53
33. Lubicz B, Van der Elst O, Collignon L, Mine B, Alghamdi F. Silk flow-diverter stent for the treatment of intracranial aneurysms: A series of 58 patients with emphasis on long-term results. AJNR Am J Neuroradiol. 2015. 36: 542-6
34. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery. 2009. 64: 632-42
35. Martínez-Galdámez M, Biondi A, Kalousek V, Pereira VM, Ianucci G, Gentric JC. Periprocedural safety and technical outcomes of the new Silk Vista Baby flow diverter for the treatment of intracranial aneurysms: Results from a multicenter experience. J Neurointerv Surg. 2019. 11: 723-7
36. Martínez-Galdámez M, Lamin SM, Lagios KG, Liebig T, Ciceri EF, Chapot R. Periprocedural outcomes and early safety with the use of the Pipeline Flex Embolization Device with Shield Technology for unruptured intracranial aneurysms: Preliminary results from a prospective clinical study. J Neurointerv Surg. 2017. 9: 772-6
37. Mattingly T, Van Adel B, Dyer E, Lopez-Ojeda P, Pelz DM, Lownie SP. Failure of aneurysm occlusion by flow diverter: A role for surgical bypass and parent artery occlusion. J Neurointerv Surg. 2015. 7: e13
38. McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P. The barrow ruptured aneurysm trial. J Neurosurg. 2012. 116: 135-44
39. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002. 360: 1267-74
40. Murthy SB, Shah S, Shastri A, Venkatasubba Rao CP, Bershad EM, Suarez JI. The SILK flow diverter in the treatment of intracranial aneurysms. J Clin Neurosci. 2014. 21: 203-6
41. Mut F, Cebral JR. Effects of flow-diverting device oversizing on hemodynamics alteration in cerebral aneurysms. AJNR Am J Neuroradiol. 2012. 33: 2010-6
42. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011. 32: 34-40
43. O’kelly CJ, Krings T, Fiorella D, Marotta TR. A novel grading scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. 2010. 16: 133-7
44. Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S. The fate of incomplete stent apposition with drug-eluting stents: An optical coherence tomography-based natural history study. Eur Heart J. 2010. 31: 1470-6
45. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: Current status. Stroke. 2013. 44: 2046-54
46. Raymond J, Gentric JC, Darsaut TE, Iancu D, Chagnon M, Weill A. Flow diversion in the treatment of aneurysms: A randomized care trial and registry. J Neurosurg. 2017. 127: 454-62
47. Raymond J, Guilbert F, Metcalfe A, Gévry G, Salazkin I, Robledo O. Role of the endothelial lining in recurrences after coil embolization: Prevention of recanalization by endothelial denudation. Stroke. 2004. 35: 1471-5
48. Rice H, Martínez Galdámez M, Holtmannspötter M, Spelle L, Lagios K, Ruggiero M. Periprocedural to 1-year safety and efficacy outcomes with the Pipeline Embolization Device with Shield technology for intracranial aneurysms: A prospective, post-market, multi-center study. J Neurointerv Surg. 2020. 12: 1107-12
49. Rouchaud A, Ramana C, Brinjikji W, Ding YH, Dai D, Gunderson T. Wall Apposition is a key factor for aneurysm occlusion after flow diversion: A histologic evaluation in 41 rabbits. AJNR Am J Neuroradiol. 2016. 37: 2087-91
50. Saake M, Struffert T, Goelitz P, Ott S, Seifert F, Ganslandt O. Angiographic CT with intravenous contrast agent application for monitoring of intracranial flow diverting stents. Neuroradiology. 2012. 54: 727-35
51. Sanchez-Recalde A, Moreno R, Barreales L, Rivero F, Galeote G, Jimenez-Valero S. Risk of late-acquired incomplete stent apposition after drug-eluting stent versus bare-metal stent. A meta-analysis from 12 randomized trials. J Invasive Cardiol. 2008. 20: 417-22
52. Simgen A, Ley D, Roth C, Yilmaz U, Körner H, MühlBenninghaus R. Evaluation of a newly designed flow diverter for the treatment of intracranial aneurysms in an elastase-induced aneurysm model, in New Zealand white rabbits. Neuroradiology. 2014. 56: 129-37
53. Sivasankar R, Shrivastava M, Limaye US. Experience with FRED junior flow diverter in treatment of cerebral aneurysms at or distal to the circle of Willis. J Clin Neurosci. 2019. 69: 166-9
54. Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Nakaji P. Ten-year analysis of saccular aneurysms in the Barrow Ruptured Aneurysm Trial. J Neurosurg. 2019. 132: 771-6
55. Struffert T, Kloska S, Engelhorn T, Deuerling-Zheng Y, Ott S, Doelken M. Optimized intravenous Flat Detector CT for non-invasive visualization of intracranial stents: First results. Eur Radiol. 2011. 21: 411-8
56. Szikora I, Berentei Z, Kulcsar Z, Barath K, Berez A, Bose A. Endovascular treatment of intracranial aneurysms with parent vessel reconstruction using balloon and self expandable stents. Acta Neurochir (Wien). 2006. 148: 711-23
57. Szikora I, Berentei Z, Kulcsar Z, Marosfoi M, Vajda ZS, Lee W. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol. 2010. 31: 1139-47
58. Turowski B, Macht S, Kulcsár Z, Hänggi D, Stummer W. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): Do we need to rethink our concepts?. Neuroradiology. 2011. 53: 37-41
59. van der Marel K, Gounis MJ, Weaver JP, de Korte AM, King RM, Arends JM. Grading of Regional Apposition after Flow-Diverter Treatment (GRAFT): A comparative evaluation of VasoCT and intravascular OCT. J Neurointerv Surg. 2016. 8: 847-52
60. Wu Q, Shao Q, Li L, Liang X, Chang K, Li T. Prophylactic administration of tirofiban for preventing thromboembolic events in flow diversion treatment of intracranial aneurysms. J Neurointerv Surg. 2021. 13: 835-40