- Department of Neurosurgery, University of California, David Geffen School of Medicine, California, USA
- Miller School of Medicine, University of Miami, Miami, Florida, USA
Correspondence Address:
Daniel Diaz-Aguilar
Department of Neurosurgery, University of California, David Geffen School of Medicine, California, USA
DOI:10.4103/sni.sni_475_17
Copyright: © 2018 Surgical Neurology International This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.How to cite this article: Daniel Diaz-Aguilar, Sergei Terterov, Alexander M. Tucker, Shaina Sedighim, Rudi Scharnweber, Stephanie Wang, Catherine Merna, Shayan Rahman. Simultaneous cerebrospinal fluid and hematologic metastases in a high-grade ependymoma. 26-Apr-2018;9:93
How to cite this URL: Daniel Diaz-Aguilar, Sergei Terterov, Alexander M. Tucker, Shaina Sedighim, Rudi Scharnweber, Stephanie Wang, Catherine Merna, Shayan Rahman. Simultaneous cerebrospinal fluid and hematologic metastases in a high-grade ependymoma. 26-Apr-2018;9:93. Available from: http://surgicalneurologyint.com/surgicalint-articles/simultaneous-cerebrospinal-fluid-and-hematologic-metastases-in-a-high%e2%80%91grade-ependymoma/
Abstract
Background:Ependymomas are relatively uncommon tumors that constitute about 7% of all primary intracranial neoplasms. Among these, high-grade ependymomas are locally aggressive and recur most commonly at the primary site following resection. Ependymomas are also known to be the one glial neoplasm that tends to frequently metastasize inside and outside the central nervous system (CNS) that complicates workup and management. Metastasis due to surgical manipulation is common and neurosurgeons should be well-versed in the most effective methods to remove these tumors in order to avoid such metastases.
Case Description:Here, we report a case of a 28-year-old female who initially presented with a parenchymal World Health Organization (WHO) grade III anaplastic ependymoma of the occipital lobe without metastasis. After multiple resections, the patient showed no evidence of disease recurrence for 2 years. During follow-up, new metastasis to the frontal lobe as well as to the lung were discovered 2 years after the initial surgery, without recurrence at the tumor's primary site.
Conclusions:While uncommon, this case demonstrates the possibility for ependymomas to metastasize via cerebrospinal fluid to other locations within the CNS and hematologically to extraneural locations without recurring locally.
Keywords: Cerebrospinal fluid metastasis, ependymoma, hematologic metastasis
INTRODUCTION
Ependymomas are primary glial cell tumors that arise from ependymal cells lining the ventricular system.[
At present, the primary therapeutic intervention for intracranial ependymomas is surgery.[
The majority of ependymomas are not located in the parenchyma but located within the cerebrospinal fluid (CSF) pathways and have a relatively high rate of metastasis compared to other brain tumors. Workup for these neoplasms, therefore, includes imaging of the entire cranio-spinal axis and between 8 and 20% of high-grade ependymomas demonstrate CSF spread at presentation.[
Here, we report a case of a 28-year-old female who initially presented with a WHO grade III anaplastic ependymoma of the occipital lobe without any signs of metastasis. After multiple resections, which were required for the index site, the patient showed no evidence of disease recurrence in the CNS for 2 years. At the 2-year follow-up appointment, new metastasis to the frontal lobe and lung were discovered in the absence of any recurrence at the tumor's primary site.
The case presented is of particular interest for multiple reasons. First, it is highly uncommon for an ependymoma to metastasize in retrograde to the primary lesion. Second, concurrent brain and lung metastases suggest sequential CSF and hematologic spread, which, to our knowledge, has yet to be reported within the literature.
CASE REPORT
A 28-year-old female presented to an outside hospital in 2007 with fainting spells that were suspicious for seizures by family's report. A magnetic resonance imaging (MRI) was obtained and revealed a right occipital tumor. The patient underwent a right occipital craniotomy and image-guided resection of the lesion. It was noted that there were three areas which were concerning for tumor invasion of the surrounding parenchyma due to its general discoloration. Frozen biopsies were sent for evaluation. The pathology report came back negative for marginal tumor infiltration. The patient was incorrectly told that the tumor was meningioma due to an incorrect preliminary read and that no further treatment was necessary; however, the final pathological diagnosis revealed a WHO grade III anaplastic ependymoma. Unfortunately, the revised diagnosis was not made known to the patient, nor her future physicians. She did not undergo chemotherapy or radiation at that time.
In 2012, the patient presented to our hospital with severe (8/10) headaches, nausea, and photophobia. Computed tomography (CT) and MRI of the brain did not show any acute pathology or evidence of tumor recurrence. The patient was given a diagnosis of migraine headaches and treated medically.
The patient re-presented in 2014 with relapse of her headaches and fainting spells. An MRI of the brain was obtained, revealing a recurrent 4.1 cm enhancing mass in the right occipital lobe with surrounding edema (without evidence of drop metastasis or other enhancing lesions on spinal MRI [
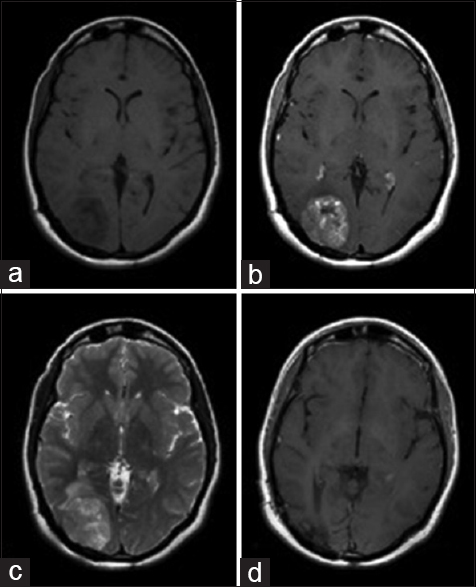
Figure 1
Preoperative axial MR images showing an enhancing mass in the occipital lobe with isointensity on T1-weighted images (a), hyper intensity on a gadolinium enhanced T1-weighted image (b), and hyper intensity on T2-weighted image (c). Postoperative axial gadolinium enhanced T1-weighted image demonstrating complete resection of the mass with no regions of hyper intensity (d)
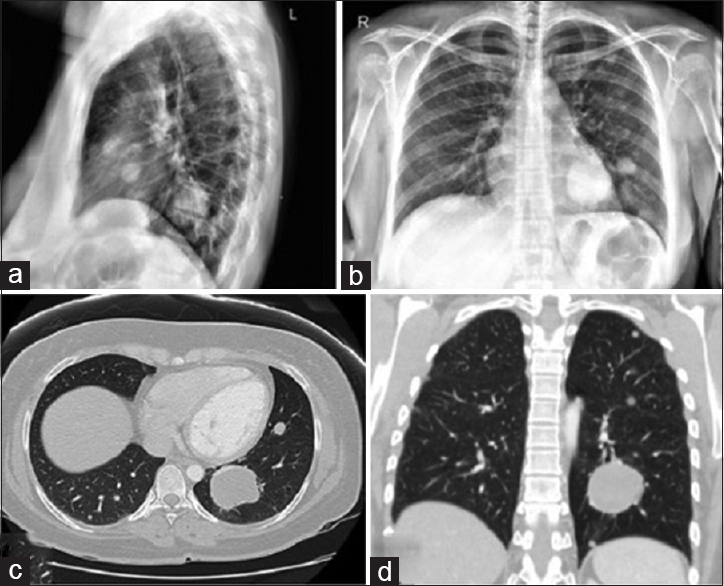
Two years after her second resection, the patient presented to her primary care physician with a cough lasting for several weeks. A chest X-ray was performed, which revealed multiple, bilateral pulmonary nodules. The largest lesion was seen in the left lower lobe, measuring 4.5 × 3.4 × 3.9 cm [
An MRI of the brain was obtained at the same time, which revealed the interval development of several new right-sided intracranial frontal extraaxial masses side, but without any evidence of tumor recurrence in the right occipital lobe tumor resection bed [Figure
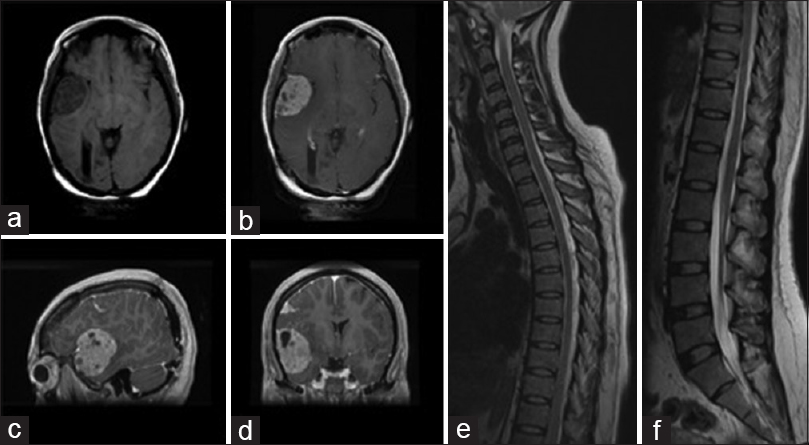
Figure 3
Two year post operative MR axial images demonstrating T1 weighted a non enhancing fronto-temporal mass (a), with hyperintensity following gadolinium enhancement on T1-weighted axial (b), sagittal (c) and coronal views (d). Images demonstrating the absence of drop metastases or other enhancing lesions T1-weighted gadolinium enhanced images of thoracic (e), and lumbar (f) spinal cord
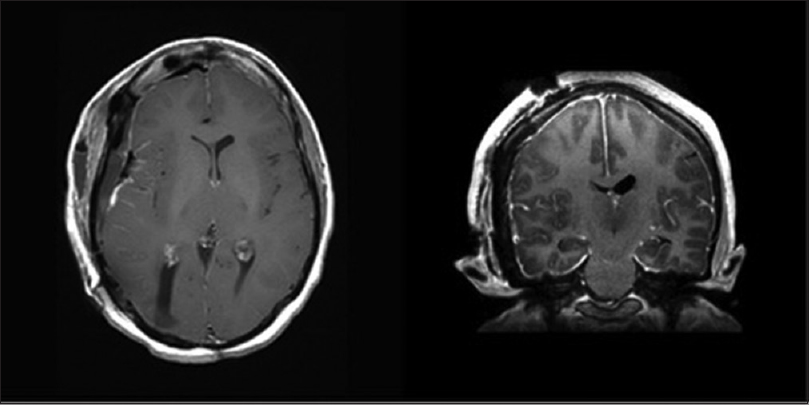
The patient once again underwent respective surgery and all cranial tumors were removed successfully as seen on postoperative imaging [
The patient is currently under close follow-up, with adjuvant radiotherapy and chemotherapy for her lung metastases without evidence of intracranial recurrence 12 months following her most recent resection seen on imaging.
DISCUSSION
Ependymomas are primary tumors of the CNS that primarily affect children and young adults.[
The scenario of intracranial tumors showing metastasis to an extraneural location has been intensely studied, but the mechanism of this spread of disease is not yet fully understood.[
Extracranial ependymoma metastases are exceedingly rare, and are most often found at the time of primary tumor recurrence.[
Direct surgical manipulation of the tumor and CSF-shunt placement are potent avenues for intra- and extraneural metastasis formation in these cases. The physical disruption of the blood–brain barrier during surgery, combined with the dislodging of malignant cells into local structures, following direct tumor manipulation, is likely to play a role in the formation of about 8.5% of extracranial metastases.[
High-grade ependymomas are locally aggressive and recur most commonly at the primary site following resection. They are also known to be the glial tumor most prone to metastasis. Malignant cells have been identified within the CSF, drawn in the typical fashion of three vials of 2 cc. On average, 16% of patients with known ependymomas.[
Pulmonary metastases, on the contrary, are likely the result of hematogenous spread via tumor cells that gained access to the circulation during surgery. Such CSF seeding to extracranial sites is possible via mechanical disruption of the blood–brain barrier at the time of surgery allowing tumor cell to reach the venous system, which then permits hematogenous spread to the lung. Similarly, it is possible that the intracranial tumor could have spontaneously invaded either vascular or lymphatic structures, leading to seeding of tumor cells to the lungs.
While uncommon, and not extensively written about in the literature, this case demonstrates the possibility for ependymomas to simultaneously metastasize both through CSF to other CNS locations and hematologically to extraneural locations. Such pathways should be considered when routinely monitoring patients for tumor growth or recurrence.
CONCLUSION
Ependymomas rarely metastasize without local recurrence. To the best of our knowledge, our case is the first report on anaplastic ependymoma with concurrent CSF and hematologic metastasis, occurring without recurrence at the primary site. This unusual pattern of neoplasm recurrence prompts a discussion of the pathogenesis of extraneural metastasis and highlights a possible risk of surgical intervention.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Alzahrani A, Alassiri A, Kashgari A, Alrehaili J, Alshaalan H3, Zakzouk R4. Extraneural metastasis of an ependymoma: A rare occurrence. Neuroradiol J. 2014. 27: 175-8
2. Ambekar S, Ranjan M, Prasad C, Santosh V, Somanna S. Fourth ventricular ependymoma with a distant intraventricular metastasis: Report of a rare case. J Neurosci Rural Pract. 2013. 4: S121-4
3. Brandao LA, Young Poussaint T. Posterior fossa tumors. Neuroimaging Clin N Am. 2017. 27: 1-37
4. Chao MM, Packer RJ, Myseros JS, Rood BR. Isolated extracranial recurrence of anaplastic ependymoma. Pediatr Blood Cancer. 2011. 56: 317-8
5. Davis MJ, Hasan F, Weinreb I, Wallace MC, Kiehl TR. Extraventricular anaplastic ependymoma with metastasis to scalp and neck. J Neurooncol. 2011. 104: 599-604
6. Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. 2012. 14: v1-49
7. Duffner PK, Cohen ME. Extraneural metastases in childhood brain tumors. Ann Neurol. 1981. 10: 261-5
8. Fischer C, Haque SS, Huse JT, Blochin E, Souweidane MM, Lis E. Extraneural ependymoma: Distant bone, lung, liver, and lymph node metastases following bevacizumab. Pediatr Blood Cancer. 2013. 60: 143-5
9. Gilbert MR, Ruda R, Soffietti R. Ependymomas in adults. Curr Neurol Neurosci Rep. 2010. 10: 240-7
10. Godfraind C. Classification and controversies in pathology of ependymomas. Childs Nerv Syst. 2009. 25: 1185-93
11. Gramatzki D, Roth P, Felsberg J, Hofer S, Rushing EJ, Hentschel B. Chemotherapy for intracranial ependymoma in adults. BMC Cancer. 2016. 16: 287-
12. Itoh J, Usui K, Itoh M, Hashizume Y. Extracranial metastases of malignant ependymoma--case report. Neurol Med Chir (Tokyo). 1990. 30: 339-45
13. Jung J, Choi W, Ahn SD, Park JH, Kim SS, Kim YS. Postoperative radiotherapy for ependymoma. Radiat Oncol J. 2012. 30: 158-64
14. Kinoshita M, Izumoto S, Kagawa N, Hashimoto N, Maruno M, Yoshimine T. Long-term control of recurrent anaplastic ependymoma with extracranial metastasis: Importance of multiple surgery and stereotactic radiosurgery procedures—case report. Neurol Med Chir (Tokyo). 2004. 44: 669-73
15. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114: 97-109
16. Mavroudis C, Townsend JJ, Wilson CB. A metastasizing ependymoma of the cauda equina. Case report. J Neurosurg. 1977. 47: 771-5
17. Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park). 2002. 16: 629-42
18. Merchant TE. Current clinical challenges in childhood ependymoma: A focused review. J Clin Oncol. 2017. 35: 2364-9
19. Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017. 28: 228-43
20. Murakami M, Kuratsu J, Takeshima H, Soyama N, Shinojima N, Ushio Y. Spinal seeding of anaplastic ependymoma mimicking fungal meningitis. A case report and review of the literature. J Neurosurg Sci. 2000. 44: 46-51
21. Newton HB, Henson J, Walker RW. Extraneural metastases in ependymoma. J Neurooncol. 1992. 14: 135-42
22. Pollack IF, Gerszten PC, Martinez AJ, Lo KH, Shultz B, Albright AL. Intracranial ependymomas of childhood: Long-term outcome and prognostic factors. Neurosurgery. 1995. 37: 655-
23. Ramaswamy V, Taylor MD. Treatment implications of posterior fossa ependymoma subgroups. Chin J Cancer. 2016. 35: 93-
24. Severino M, Consales A, Doglio M, Tortora D, Morana G, Barra S. Intradural extramedullary ependymoma with leptomeningeal dissemination: The first case report in a child and literature review. World Neurosurg. 2015. 84: 865 e813-869
25. Shim KW, Kim DS, Choi JU. The history of ependymoma management. Childs Nerv Syst. 2009. 25: 1167-83
26. Vera-Bolanos E, Aldape K, Yuan Y, Wu J, Wani K, Necesito-Reyes MJ. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015. 17: 440-7
27. Wight DG, Holley KJ, Finbow JA. Metastasizing ependymoma of the cauda equina. J Clin Pathol. 1973. 26: 929-35
28. Wood H. Neuro-oncology: A new approach to ependymoma subtyping. Nat Rev Neurol. 2017. 13: 512-3
29. Wu J, Armstrong TS, Gilbert MR. Biology and management of ependymomas. Neuro Oncol. 2016. 18: 902-13
30. Yao Y, Mack SC, Taylor MD. Molecular genetics of ependymoma. Chin J Cancer. 2011. 30: 669-81
31. Zhang XP, Liu Y, Zhang D, Zheng Q, Wang C, Wang L. Cerebellar ependymoma with overlapping features of clear-cell and tanycytic variants mimicking hemangioblastoma: A case report and literature review. Diagn Pathol. 2017. 12: 28-
32. Zhao C, Wang C, Zhang M, Jiang T, Liu W, Li W. Primary cerebellopontine angle ependymoma with spinal metastasis in an adult patient: A case report. Oncol Lett. 2015. 10: 1755-58