- Department of Neurology, American University of Beirut Medical Center, Beirut, Lebanon.
- Department of Neurosurgery, American University of Beirut Medical Center, Beirut, Lebanon.
Correspondence Address:
Ahmad Beydoun, Department of Neurology, American University of Beirut Medical Center, Beirut, Lebanon.
DOI:10.25259/SNI_946_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Sahar Farhat1, Jawad Melhem1, Houssein Darwish2, Shadi Bsat2, Sarah Kawtharani2, Hiba Fadlallah2, Marwan Najjar2, Ahmad Beydoun1. Simultaneous sampling of both cingulate gyri using a single interhemispheric depth electrode: A technical note. 12-Jul-2024;15:242
How to cite this URL: Sahar Farhat1, Jawad Melhem1, Houssein Darwish2, Shadi Bsat2, Sarah Kawtharani2, Hiba Fadlallah2, Marwan Najjar2, Ahmad Beydoun1. Simultaneous sampling of both cingulate gyri using a single interhemispheric depth electrode: A technical note. 12-Jul-2024;15:242. Available from: https://surgicalneurologyint.com/surgicalint-articles/12987/
Abstract
Background: Simultaneous sampling of the cingulate gyri through a single depth electrode inserted underneath the falx cerebri is clinically useful in certain cases of drug-resistant epilepsy. However, the frequency at which each region of the cingulate gyri – namely, anterior, middle, and posterior – can be simultaneously sampled with a single electrode remains uncertain.
Methods: We assessed the anatomical relationship between the falx cerebri and the cingulate gyrus in 50 adults and children. Subsequently, we determined whether an arbitrary line, denoted as A (representing a 5 mm gap between the falx cerebri and corpus callosum necessary for depth electrode insertion), fell within the anterior, middle, or posterior cingulate gyrus.
Results: The shape of the falx cerebri and its intersection point with the corpus callosum varied substantially across individuals, with a significant difference between children and adults (P = 0.02). The A line was located in the middle cingulate gyrus in 18 children (72%), while 3 (12%) and 4 (16%) had it located in the posterior and anterior cingulate gyrus, respectively. Among adults, 15 individuals (60%) had the A line in the middle cingulate gyrus, 10 (40%) in the posterior cingulate gyrus, and none in the anterior cingulate gyrus.
Conclusion: This study demonstrates the feasibility of simultaneous sampling of both the anterior and middle cingulate gyri in adults and children. Moreover, it represents the first investigation to document the wide interindividual variability in the morphology of the falx cerebri and its association with the cingulate gyrus.
Keywords: Epilepsy, Cingulate gyrus, Depth electrode, Stereoelectroencephalography, Sampling, Falx cerebri
INTRODUCTION
The cingulate gyrus lies on the medial aspect of the brain, forming a belt above the corpus callosum. Due to its vast connections with the frontal, temporal, parietal, occipital, and insular cortices, the semiology of seizures originating from the cingulate gyrus is diverse and frequently reflects ictal propagation to associated symptomatogenic areas.[
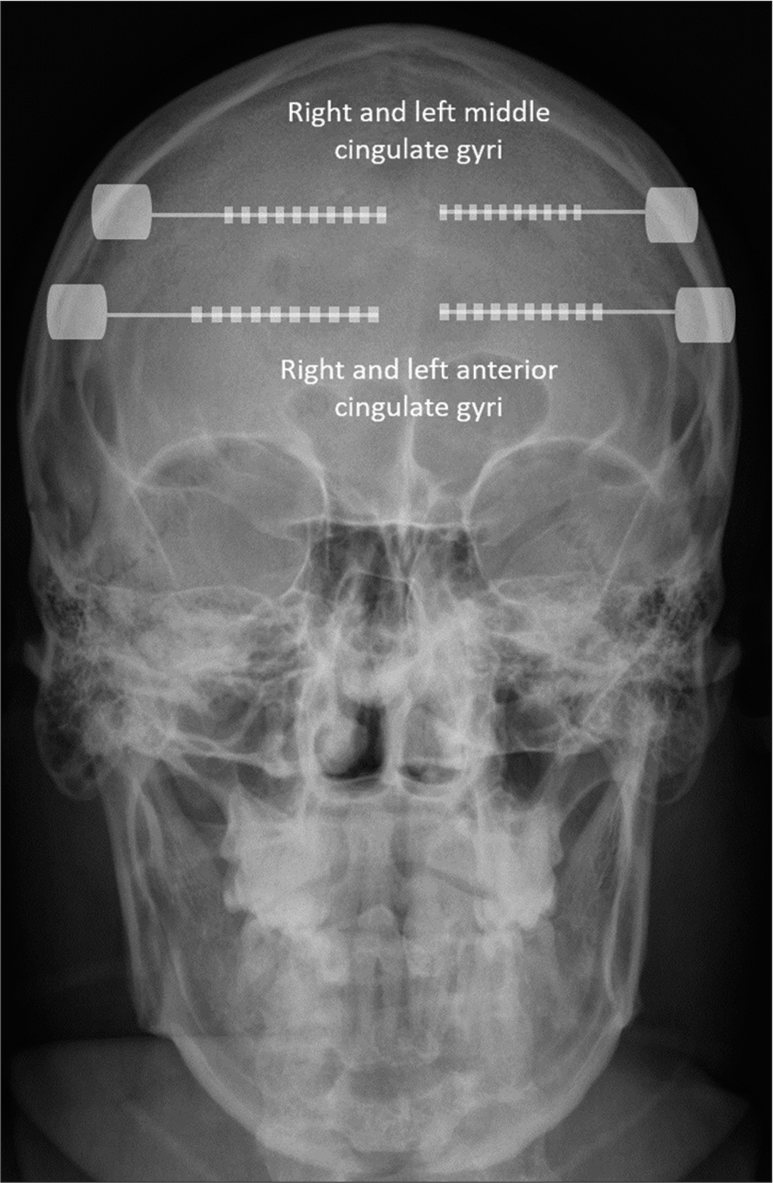
When the lateralization of the seizure onset zone is unknown, and both cingulate gyri need sampling, the current technique involves placing SEEG depth electrodes in each hemisphere with an orthogonal orientation in relation to the sagittal plane, targeting the anterior, mid, and/or posterior cingulate gyri based on the presurgical hypothesis [
However, there are knowledge gaps regarding the feasibility of bilateral sampling of the cingulate gyri due to the unclear anatomical relationship between the falx cerebri and the cingulate gyrus. Anteriorly, where the falx only partially covers the cingulate gyrus, electrode insertion beneath the falx allows for targeted sampling of the exposed portions of the cingulate gyrus. However, as the falx extends posteriorly and covers the cingulate gyrus entirely, electrode insertion below the falx is no longer feasible. In this study, we aim to address these knowledge gaps by evaluating the anatomical relationship between the falx cerebri and the cingulate gyrus in 50 adults and children to determine how frequently the anterior, middle, and posterior cingulate gyri could be sampled with this technique.
MATERIALS AND METHODS
Anatomical relationship between the cingulate gyrus and falx cerebri
We reviewed the epilepsy protocol brain magnetic resonance images (MRIs) of 50 subjects (25 children and 25 adults) with normal neuroimaging participating in an ongoing study approved by the Institutional Review Board of the American University of Beirut Medical Center evaluating patients with suspected new onset seizure/epilepsy. The brain MRIs were obtained from a 1.5 or 3T scanner (Ingenia; Phillips Healthcare) using an imaging-acquisition protocol that included 3D T1 (1 mm slice thickness) and 3D fast fluid-attenuated inversion recovery (0.9 or 1 mm slice thickness) of the whole brain with multiplanar reconstruction, axial and coronal inversion recovery (2 mm slice thickness), axial T2 turbo spin echo (TSE) and T2 fast field echo (FFE) (4 mm slide thickness), and axial diffusion weighted images (4–5 mm slice thickness). The 3D images were obtained with no interslice gap. All subjects or their parents signed an informed consent.
We used the T1 midsagittal plane to calculate how frequently the anterior, middle, and posterior cingulate cortices could be simultaneously sampled through depth electrodes inserted beneath the falx cerebri. Based on the Talairach and Tournoux coordinates,[ I point: Represents the point of intersection of the falx cerebri with the corpus callosum [ A line represents the line with a 5 mm opening between the inferior border of the falx cerebri and the superior border of the corpus callosum. This line was drawn perpendicular to a tangent line to the surface of the corpus callosum [
Figure 2:
Anatomical relationship between the falx cerebri and the corpus callosum. The anterior cingulate gyri, middle cingulate gyri, and posterior cingulate gyri were divided according to their location anterior to the vertical anterior commissure line (VAC), in between the VAC and vertical posterior commissure (VPC) lines and posterior to the VPC, respectively. The I point represents the intersection between the falx cerebri and the corpus callosum, while the A line represents the location with a gap of 5 mm between the inferior border of the falx cerebri and the superior edge of the corpus callosum. The free edge of the falx cerebri points to the inferior sagittal sinus. AC: Anterior commissure, PC: Posterior commissure, ACG: anterior cingulate gyrus, MCG: middle cingulate gyrus, PCG: posterior cingulate gyrus.
RESULTS
There were substantial variabilities in the shape of the falx cerebri and the point where it abutted with the corpus callosum. For some subjects [
Figure 3:
Sagittal magnetic resonance imaging cuts showing the variable anatomy of the falx cerebri in relation to the corpus callosum. In some subjects (a), the A line was located in the posterior cingulate gyrus (PCG), allowing bilateral sampling of the anterior cingulate gyri (ACG), middle cingulate gyri (MCG) and PCG. In most subjects (b), the A line was located in the MCG, allowing sampling of the ACG and MCG. In a small percentage of children (c), the A line was located in the ACG, therefore only allowing for both ACG to be simultaneously sampled with this technique, VAC: Vertical anterior commissure line, VPC: Vertical posterior commissure line, AC: Anterior commissure, PC: Posterior commissure.
The demographics and locations of the A line for the 50 subjects are shown in
For the whole cohort, 33 subjects (66%) had the A line located in the middle cingulate gyrus, 13 (26%) had it located in the posterior cingulate gyrus, and only 4 (8%) had it located in the anterior cingulate gyrus [
DISCUSSION
In this study, we evaluated the anatomical relationship between the falx cerebri and the cingulate gyrus in 50 adults and children to determine how frequently the anterior, middle, and posterior cingulate gyri could be bilaterally sampled using a single interhemispheric depth electrode inserted from one hemisphere, directed toward the ipsilateral cingulate gyrus and advanced underneath the falx cerebri to reach the contralateral gyrus.
This is the first study to analyze the relationship of the falx cerebri to the cingulate gyrus. We showed that despite the wide interindividual variability in the location of the A point, this approach can be used to simultaneously monitor the anterior and middle cingulate gyri in most adults and children. Of particular interest were the age-related anatomical differences identified in our study, highlighting the importance of considering developmental factors when planning depth electrode placements. We observed significant variations in the VAC-VPC distance and the anatomical location of the A line between adult and pediatric populations, underscoring the need for age-specific approaches to electrode insertion. In the adult population, our data showed that this technique enables sampling of the anterior and middle cingulate gyri in all patients and that all three divisions of the cingulate gyrus can be assessed in 40% of patients. Conversely, the anterior and middle cingulate gyri can be sampled in 72% of children, while all three divisions can be evaluated in 12% of children. In 16% of children, only the anterior cingulate gyrus can be sampled bilaterally.
In patients with medically refractory epilepsy, bilateral sampling of the cingulate gyri may be necessary, particularly when a presurgical evaluation suggests an ictal onset in the mesial frontal lobe without evident epileptogenic lesions on brain MRIs.[
A recent study investigated the safety and utility of bihemispheric sampling through transmidline SEEG in 53 patients who had 83 electrodes implanted in the anterior and middle divisions of the cingulate gyri.[
CONCLUSION
Depth electrodes inserted into the cingulate gyrus, oriented orthogonally relative to the sagittal plane, and directed toward the contralateral gyrus by insertion beneath the falx cerebri allow for the exploration of homologous cingulate regions in both hemispheres with a single rather than a pair of electrodes. This technique is safe and enables sampling of the anterior and middle cingulate gyri in all adult patients and nearly three quarters of pediatric patients, thus reducing the required number of electrodes and lowering both the procedural risks and costs.
Ethical approval
The Institutional Review Board has waived the ethical approval for this study.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Alkawadri R, So NK, Van Ness PC, Alexopoulos AV. Cingulate epilepsy: Report of 3 electroclinical subtypes with surgical outcomes. JAMA Neurol. 2013. 70: 995-1002
2. Chassoux F, Navarro V, Catenoix H, Valton L, Vignal JP. Planning and management of SEEG. Neurophysiol Clin. 2018. 48: 25-37
3. Chou CC, Lee CC, Lin CF, Chen YH, Peng SJ, Hsiao FJ. Cingulate gyrus epilepsy: Semiology, invasive EEG, and surgical approaches. Neurosurg Focus. 2020. 48: E8
4. D’Orio P, Revay M, Bevacqua G, Battista F, Castana L, Squarza S. Stereo-electroencephalography (SEEG)-guided surgery in epilepsy with cingulate gyrus involvement: Electrode implantation strategies and postoperative seizure outcome. J Clin Neurophysiol. 2023. 40: 516-28
5. Gonzalez-Martinez J, Mullin J, Vadera S, Bulacio J, Hughes G, Jones S. Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg. 2014. 120: 639-44
6. Guenot M, Isnard J, Ryvlin P, Fischer C, Ostrowsky K, Mauguiere F. Neurophysiological monitoring for epilepsy surgery: The Talairach SEEG method. Stereotact Funct Neurosurg. 2001. 77: 29-32
7. Inoyama K, Devinsky O. Cingulate seizures and recent treatment strategies. Handb Clin Neurol. 2019. 166: 341-53
8. Isnard J, Taussig D, Bartolomei F, Bourdillon P, Catenoix H, Chassoux F. French guidelines on stereoelectroencephalography (SEEG). Neurophysiol Clin. 2018. 48: 5-13
9. Luders HO, editors. Textbook of epilepsy surgery. United States: CRC Press; 2008. p.
10. Restrepo CE, Balaguera P, Thompson SA, Johnson J, Lacuey N, Pati S. Safety and efficacy of bihemispheric sampling via transmidline stereoelectroencephalography. J Neurosurg. 2022. 139: 229-37
11. Talairach J, Tournoux P, editors. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. p.
12. Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003. 18: 3134-44