- School of Medicine, University of Pittsburgh, Pennsylvania, United States
- Department of Neurosurgery, University of Pittsburgh, Medical School, Pennsylvania, United States
- Department of Orthopaedic Surgery Pittsburgh, Pennsylvania, United States.
Correspondence Address:
Nikhil Sharma, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, United States.
DOI:10.25259/SNI_894_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Nikhil Sharma1, Jeffery R. Head2, Arka N. Mallela2, Regan M. Shanahan1, Stephen P. Canton3, Hussam Abou-Al-Shaar2, Nicolás Matheo Kass1, Fritz Steuer1, Lucille Cheng1, Michael Raver1, Edward G. Andrews2. Single institution series describing external ventricular drain (EVD) placement and short- and long-term complications related to placement accuracy. 01-Mar-2024;15:67
How to cite this URL: Nikhil Sharma1, Jeffery R. Head2, Arka N. Mallela2, Regan M. Shanahan1, Stephen P. Canton3, Hussam Abou-Al-Shaar2, Nicolás Matheo Kass1, Fritz Steuer1, Lucille Cheng1, Michael Raver1, Edward G. Andrews2. Single institution series describing external ventricular drain (EVD) placement and short- and long-term complications related to placement accuracy. 01-Mar-2024;15:67. Available from: https://surgicalneurologyint.com/surgicalint-articles/12777/
Abstract
Background: The placement of an external ventricular drain (EVD) for the treatment of acute hydrocephalus is one of the most common life-saving procedures that neurosurgeons perform worldwide. There are many well-known complications associated with EVD placement, including tract hemorrhages, intra-parenchymal and subdural hemorrhages, infection, and catheter misplacement. Given the variety of complications associated with EVD placement and the inconsistent findings on the relationship of accuracy to complications, the present study reviewed short- and long-term complications related to EVD placement at our institution.
Methods: A retrospective review was conducted for all consecutive patients who underwent bedside EVD placement for any indication between December 2020 and December 2021. Collected variables included demographic information, etiology of disease state, pre-and post-operative head computed tomography measurements, and post-procedural metrics (immediate and delayed complications).
Results: A total of 124 patients qualified for inclusion in our study. EVDs that were non-functioning/exchanged were not significantly related to age, accuracy, ventriculomegaly, sex, disposition, laterality, type of EVD used, intraventricular hemorrhage (IVH), etiology, or Kakarla Grade (KG) (all P > 0.17). The need for a second EVD was similarly not related to age, accuracy, ventriculomegaly, sex, disposition, location, laterality, type of EVD used, IVH, etiology, or KG (all P > 0.130). Patients who died, however, were significantly more likely to have a second contralateral EVD placed (18.2% vs. 4.9% P = 0.029). We also found that left-sided EVDs were significantly more likely to fail within seven days of placement (29.4% vs 13.3%, P = 0.037; relative risk (RR) 1.93, 95% confidence interval: 1.09-3.43), unrelated to age, sex, etiology, type of EVD, IVH, location of the procedure, or accuracy (all P > 0.07). This remained significant when using a binary logistic regression to control for ventriculomegaly, accuracy, mortality, age, sex, and etiology (P = 0.021, B = 3.43).
Conclusion: In our cohort, although a clear relationship between inaccuracy and complication rates was not found, our data did demonstrate that left-sided EVDs were more likely to fail within the immediate postoperative time point, and patients who died were more likely to have a second, contralateral EVD placed.
Keywords: Accuracy, Complications, External ventricular drain (EVD)
INTRODUCTION
The placement of an external ventricular drain (EVD) for the treatment of acute hydrocephalus or elevated intracranial pressure (ICP) is one of the most common life-saving procedures that neurosurgeons perform worldwide.[
There are many well-known complications associated with EVD placement, including tract hemorrhages, intraparenchymal and subdural hemorrhages, infection, and catheter misplacement.[
Kakarla et al. first studied the relationship of EVD accuracy to complications with the use of a subjective grading system for EVD placement, with the ideal position being categorized as a catheter tip in the ipsilateral frontal horn or third ventricle (Grade 1) ranging to suboptimal placement in the eloquent cortex (Grade 3).[
Given the variety of complications associated with EVD placement and the inconsistent findings on the relationship of accuracy to complications, the present study reviewed short- and long-term complications related to EVD placement at our institution.
MATERIALS AND METHODS
Patient selection
A retrospective review was conducted for all consecutive patients who underwent bedside EVD placement for any indication between December 2020 and December 2021. All EVDs were placed by members of the neurosurgical team (specifically residents) at the bedside, either in the intensive care unit (ICU) or in the emergency department, using standard procedural protocols, without neuronavigation and secured to the skin by suture. All patients received pre-procedural antibiotics (usually ancef) before receiving the EVD, as per our institution’s protocol. Patients who received antibiotic-impregnated EVDs were at the discretion of the neurosurgical team. EVDs that were antibiotic-impregnated included minocycline and rifampin. The tubing for EVDs is barium impregnated, and the dimensions are as follows: 80 cm by 1.5 mm by 0.7 mm. All EVDs at our institution are manufactured by Medtronic (Minneapolis, MN, USA). Inclusion criteria were patients who were 18 years of age or older and indicated a frontal EVD placement. Exclusion criteria were patients under the age of 18, those who received any non-frontal EVDs, and those with EVDs placed in the operating room.
Data collection and variable characteristics
This study was approved by the Institutional Review Board at the home institution, protocol number STUDY20050395. The informed consent process was waived as this was a secondary use of patient data obtained for clinical purposes. All clinical and imaging data were retrospectively collected from the patient’s electronic medical record. Collected variables included: (1) pre-procedural metrics such as demographic information, indications for EVD placement, and etiology of disease state; (2) pre- and post-operative head computed tomography (CT) measurements; (3) procedural information; and (4) post-procedural metrics (immediate and delayed complications). Etiology of hemorrhage and hydrocephalus was characterized as traumatic, subarachnoid hemorrhage (SAH), intraparenchymal hemorrhage (IPH), intraventricular hemorrhage (IVH), pseudotumor cerebri, normal pressure hydrocephalus, arteriovenous malformation, or other. Preoperative head CT measurements included the maximum width of the skull, maximum distance of the frontal horns, inter caudate distance, and maximum third ventricle distance. Post-procedural head CT measurements included the vertical distance of the tip of EVD to the foramen of Monro (FOM), the horizontal distance of the tip of EVD to the FOM, and the linear distance of the tip of EVD to the FOM (TTF). All CT measurements were completed by the first author of the manuscript and confirmed by the senior author. Procedural information was characterized by the length of the procedure, laterality of EVD placement, and Kakarla grading scale. Immediate complications were those that occurred within the present hospital stay (within one month of the EVD placement), while delayed complications were those that occurred after discharge (generally after one month). Complications were defined as tract hemorrhage, subdural hematoma, epidural hematoma, or infection. In addition, we collected data on if the EVD was revised, repositioned, removed, or replaced.
Data analysis and statistical testing
Statistical tests conducted for analysis included a Chi-squared test, paired t-test, and logistic regression. All statistical analyses were performed using SPSS software (IBM, Armonk, NY). Data are reported as mean (standard deviation) for continuous data, mean (standard deviation) for quantitative data, and n (%) for all other values. Statistically significant values were set at P < 0.05.
RESULTS
Demographics, clinical information, and operative techniques
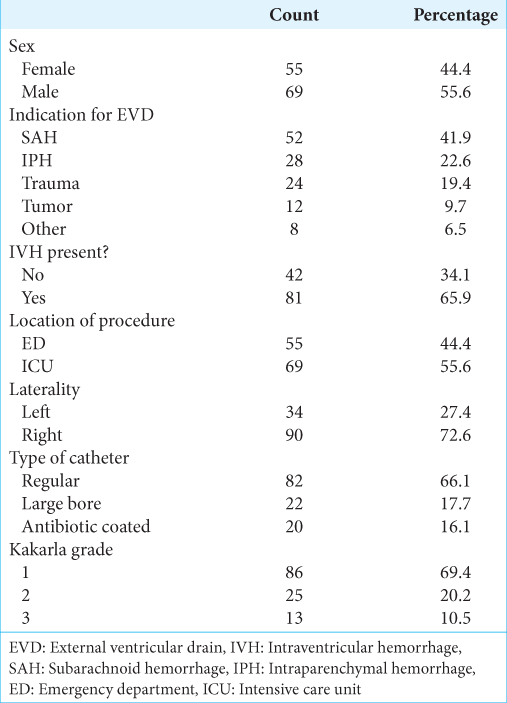
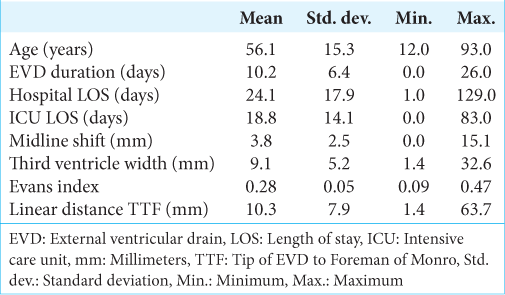
A total of 124 patients qualified for inclusion in our study. The average age was 56.1 (±15.3) years with a slight preponderance toward male patients (55.6%; n = 69) [
Most EVDs were placed in the ICU (n = 69, 55.6%), on the right side (n = 90, 72.6%), and using regular bore catheters (n = 82, 66.1%) [
Accuracy
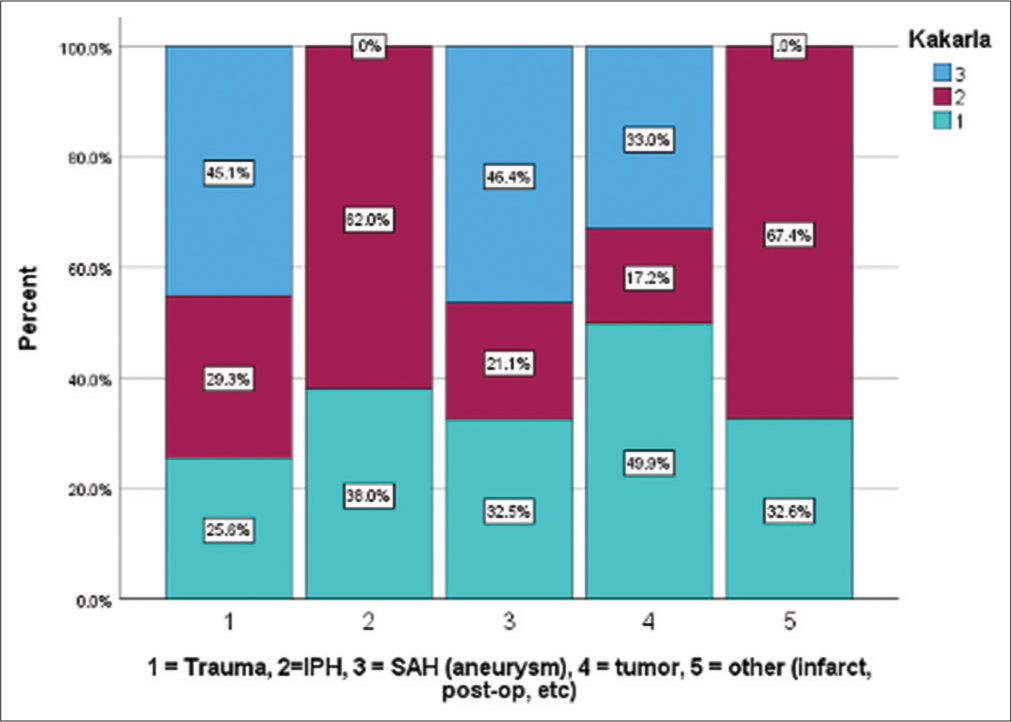
Overall, most EVDs were placed in Kakarla Grade (KG-1, n = 86, 69.4%;) [
We did find that patients without hydrocephalus (14.8% vs. 2.3%, P = 0.031) and without IVH (15.7% vs. 0%, P = 0.041) were significantly more likely to have mispositioned catheters. Patients with a misplaced EVD had significantly smaller third ventricle width (6.3 ± 3.8 vs. 9.5 ± 5.7, P = 0.037). While we thought this subset of patients might represent the trauma population, EVDs placed in traumas were not significantly more likely to have malpositioned EVDs (P = 0.271). Catheter malposition was also not related to age (P = 0.183) or sex (P = 0.467). Etiology findings related to Kakarla grade is demonstrated in
Outcomes
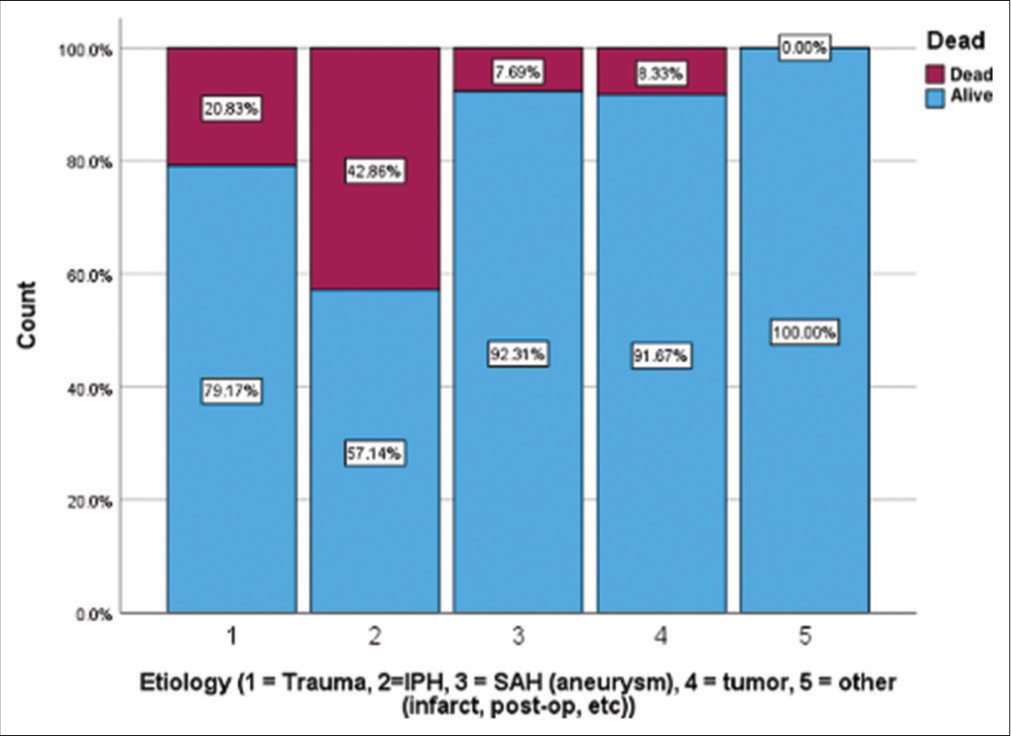
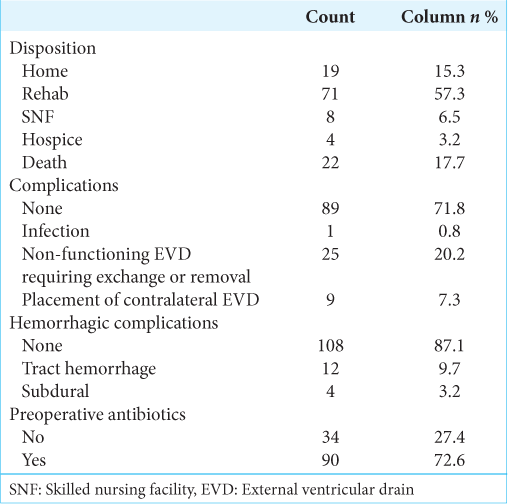
The overall rate of mortality (death or hospice) in our cohort was 17.7% (n = 22), with an average time to death of 10.45 (±8.3) days. Patients with IPH accounted for 54.5% of all deaths, which was significantly higher than any other etiology (P < 0.001), and 42.9% (n = 12) of IPH patients died [
Complications
The most common complication in our cohort was an EVD that was non-functioning or exchanged (20.2%, n = 25) followed by an EVD that required placement of a second, contralateral EVD (7.3%, n = 9) [
Overall, EVDs that were non-functioning/exchanged were not significantly related to age, accuracy, ventriculomegaly, sex, disposition, laterality, type of EVD used, IVH, etiology, or KG (all P > 0.17). The need for a second EVD was similarly not related to age, accuracy, ventriculomegaly, sex, disposition, location, laterality, type of EVD used, IVH, etiology, or KG (all P > 0.130). These comparisons all held when grouping non-functioning/exchanged EVDs with EVDs that required placement of a second, contralateral EVD. We did, however, find that patients who died were significantly more likely to have a second contralateral EVD placed (18.2% vs. 4.9% P = 0.029) but had similar rates of EVD revision (P = 0.799).
When looking at all EVD failures (non-functioning, exchanges, and second EVDs together), we found the average time to EVD failure to be 6.59 ± 5.9 days (median = 4.5 days). Therefore, we sought to analyze this subset of immediate EVD failures as defined by all EVDs that failed within the first seven days of placement. We found that left-sided EVDs were significantly more likely to fail within seven days of placement (29.4% vs 13.3%, P = 0.037; RR 1.93, 95% confidence interval: 1.09–3.43), unrelated to age, sex, etiology, type of EVD, IVH, location of the procedure, or accuracy (all P > 0.07). This remained significant when using a binary logistic regression to control for ventriculomegaly, accuracy, mortality, age, sex, and etiology (P = 0.021, B = 3.43).
DISCUSSION
Despite being one of the most common neurosurgical procedures performed worldwide, the relationship between accurate EVD placements and outcomes is not well understood in the literature. Given the ambiguity surrounding outcomes, complications, and the overall relationship to accurate EVD placements, this study sought to quantify institution-wide outcomes from free-hand EVD placements. As our institution and others investigate bedside image guidance of EVDs through augmented reality or other techniques, there is a demonstrated need to aggregate baseline data from free-hand placements for future comparisons. Despite this being an overall negative study, we did find evidence that patients who died were significantly more likely to have a second, contralateral EVD placed (18.2% vs. 4.9% P = 0.029) and left-sided EVDs were more likely to have failure rates in the immediate postoperative time point, regardless of other characteristics.
Accuracy
The rates of accuracy in this study are comparable to previous studies using the Kakarla grading scale. Within this cohort, the majority of EVDs were placed accurately (KG-1, n = 86, 69.4%); [
Outcomes
Patients with IPH experienced the highest rates of mortality. It is important to note that the placement of an EVD does not eliminate the underlying damage from the IPH event.[
Complications
The infection rate in this cohort (n = 1, 0.8%), [
Hemorrhagic complications happened more frequently in this cohort (n = 16, 12.9%), [
Limitations
Despite statistically significant results, there are limitations to our study. The most important being the nature of a single-institution and retrospective review, which limits our ability to control for certain variables, such as number of passes and time of placement as well as limits the patient population to what is seen at our institution. More so, to help overcome this selection bias, we enrolled all patients during a specific period to limit these biases.
CONCLUSION
The rates of complications due to inaccurately placed EVDs are hard to distinguish from the natural course of the disease for which EVD placement is required. In our cohort, although a clear relationship between inaccuracy and complication rates was not found, our data did demonstrate that left-sided EVDs were more likely to fail within the immediate postoperative time point, and patients who died were more likely to have a second, contralateral EVD placed. In future studies, our institution will be utilizing augmented reality image guidance to place EVDs to understand further how accuracy can impact complication rates.
Ethical approval
The research/study was approved by the Institutional Review Board at the University of Pittsburgh Medical Center, number STUDY20050395, dated 11/13/2023.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Bauer DF, Razdan SN, Bartolucci AA, Markert JM. Meta-analysis of hemorrhagic complications from ventriculostomy placement by neurosurgeons. Neurosurgery. 2011. 69: 255-60
2. Binz DD, Toussaint LG, Friedman JA. Hemorrhagic complications of ventriculostomy placement: A meta-analysis. Neurocrit Care. 2009. 10: 253-6
3. Dey M, Jaffe J, Stadnik A, Awad IA. External ventricular drainage for intraventricular hemorrhage. Curr Neurol Neurosci Rep. 2012. 12: 24-33
4. Gardner PA, Engh J, Atteberry D, Moossy JJ. Hemorrhage rates after external ventricular drain placement. J Neurosurg. 2009. 110: 1021-5
5. Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien). 2008. 150: 209-14 discussion 214
6. Kakarla UK, Kim LJ, Chang SW, Theodore N, Spetzler RF. Safety and accuracy of bedside external ventricular drain placement. Neurosurgery. 2008. 63: ONS162-6 discussion ONS166-7
7. Maher Hulou M, Maglinger B, McLouth CJ, Reusche CM, Fraser JF. Freehand frontal external ventricular drain (EVD) placement: Accuracy and complications. J Clin Neurosci. 2022. 97: 7-11
8. Maniker AH, Vaynman AY, Karimi RJ, Sabit AO, Holland B. Hemorrhagic complications of external ventricular drainage. Neurosurgery. 2006. 59: ONS419-24 discussion ONS424-5
9. Ofoma H, Cheaney B, Brown NJ, Lien BV, Himstead AS, Choi EH. Updates on techniques and technology to optimize external ventricular drain placement: A review of the literature. Clin Neurol Neurosurg. 2022. 213: 107126
10. Ramayya AG, Glauser G, McShane B, Branche M, Sinha S, Kvint S. Factors predicting ventriculostomy revision at a large academic medical center. World Neurosurg. 2019. 123: e509-14
11. Shtaya A, Roach J, Sadek AR, Gaastra B, Hempenstall J, Bulters D. Image guidance and improved accuracy of external ventricular drain tip position particularly in patients with small ventricles. J Neurosurg. 2018. 130: 1268-73
12. Stuart MJ, Antony J, Withers TK, Ng W. Systematic review and meta-analysis of external ventricular drain placement accuracy and narrative review of guidance devices. J Clin Neurosci. 2021. 94: 140-51
13. Toma AK, Camp S, Watkins LD, Grieve J, Kitchen ND. External ventricular drain insertion accuracy: Is there a need for change in practice?. Neurosurgery. 2009. 65: 1197-200 discussion 1200-1
14. Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. 1999. 27: 617-21
15. Waqar M, Chari A, Islim AI, Davies BM, Fountain DM, Larkin S. Chlorhexidine dressings could reduce external ventricular drain infections: Results from a systematic review and meta-analysis. J Hosp Infect. 2021. 117: 37-43
16. Yuen J, Selbi W, Muquit S, Berei T. Complication rates of external ventricular drain insertion by surgeons of different experience. Ann R Coll Surg Engl. 2018. 100: 221-5