- Department of Health Science, Medical School, University of Caxias do Sul, Caxias do Sul, Rio Grande do Sul, Brazil
- Department of Neurology and Neurosurgery, University of Caxias do Sul, Caxias do Sul, Rio Grande do Sul, Brazil
- Department of Medical Science, Medical School, Catholic Pontifical University of Sao Paulo, Sao Paulo, Brazil,
- Department of Neurosurgery, The Walton Centre, Liverpool, United Kingdom,
- Department of Neurology, Division of Neurology, Catholic Pontifical University of Sorocaba, Sorocaba, Brazil.
Correspondence Address:
João Pedro Einsfeld Britz, Medical School, University of Caxias do Sul, Caxias do Sul, Rio Grande do Sul, Brazil.
DOI:10.25259/SNI_372_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: João Pedro Einsfeld Britz1, Paulo Roberto Franceschini2, Miguel Bertelli Ramos1, Pedro Henrique Pires de Aguiar3, Jibril Osman Farah4, Paulo Henrique Pires de Aguiar5. Skin erosion in deep brain stimulation procedures: Using the temporalis muscle to treat this complication – A technical note. 19-Jul-2021;12:355
How to cite this URL: João Pedro Einsfeld Britz1, Paulo Roberto Franceschini2, Miguel Bertelli Ramos1, Pedro Henrique Pires de Aguiar3, Jibril Osman Farah4, Paulo Henrique Pires de Aguiar5. Skin erosion in deep brain stimulation procedures: Using the temporalis muscle to treat this complication – A technical note. 19-Jul-2021;12:355. Available from: https://surgicalneurologyint.com/surgicalint-articles/10983/
Abstract
Background: Skin erosion is a common complication after deep brain stimulator procedures. Despite being a relatively common event, there is no standard surgical technique or a widely accepted guideline for managing this kind of complication.
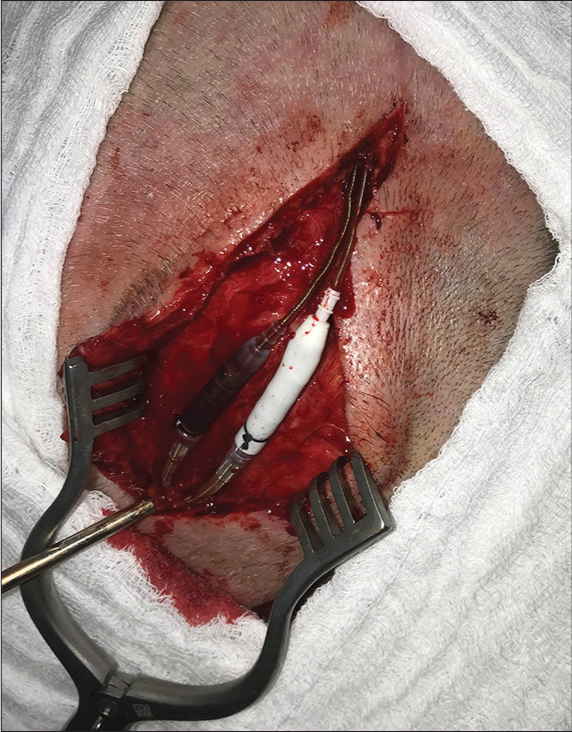
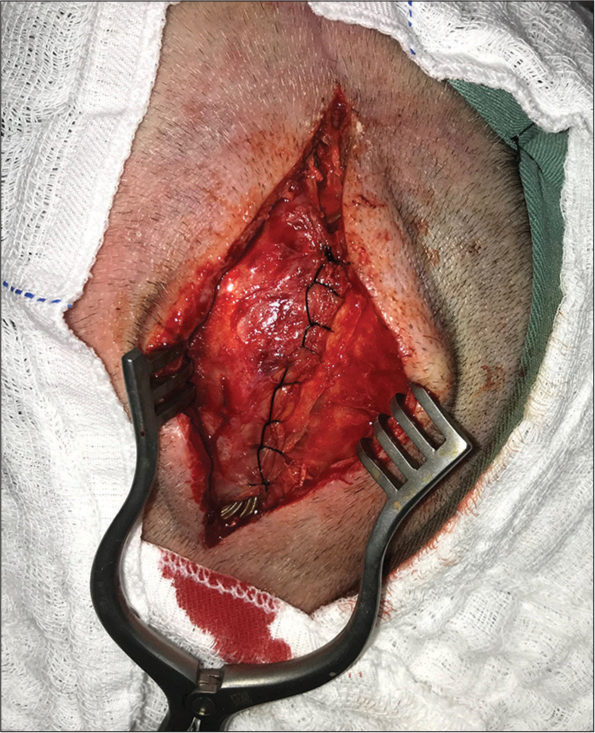
Methods: We describe a case of cutaneous erosion in the connector’s site of deep brain stimulation case, surgically managed with anterior displacement of the connectors and overlapping and wrapping the connections within the temporal muscle.
Results: Postoperatively, the patient did well and achieved complete resolution of the skin erosion, with no signs of infection or new skin lesions.
Conclusion: This technique demonstrated to be effective in this case in the long-term follow-up.
Keywords: Complications, Deep brain stimulation, Functional neurosurgery, Parkinson’s disease, Skin erosion
INTRODUCTION
Deep brain stimulation (DBS) has become increasingly common for patients with disabling symptoms due to movement disorders such as Parkinson’s disease, dystonia, and tremor.[
Skin erosion is the loss of epidermis over the implants (leads, internal pulse generator, wires, or extensions), exposing hardware and predisposing to local infections. Skin complications are the most common hardware-related complications, ranging from 1% to 15%.[
Therefore, the authors describe a case of cutaneous erosion in DBS implants that were managed using a technique not yet described in the literature.
CASE REPORT
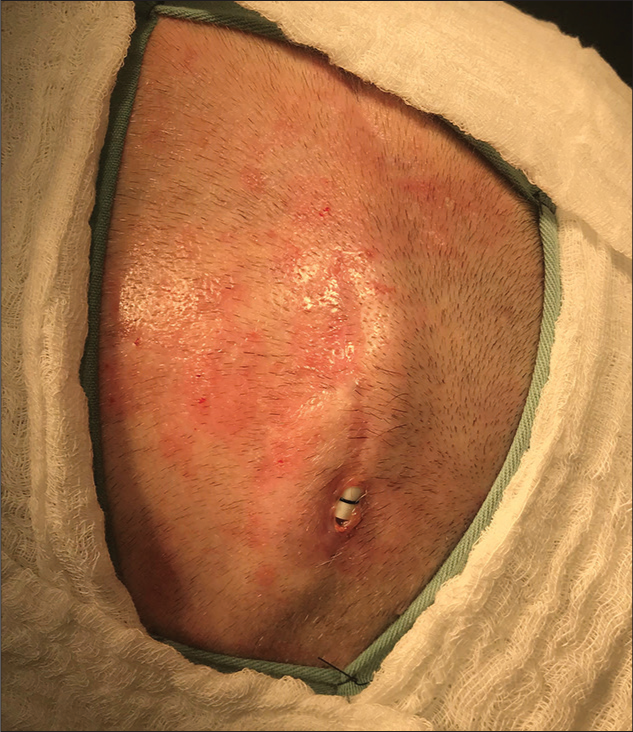
We present the case of a 62-year-old female diagnosed with Parkinson’s disease with 10 years of the onset of the first symptoms. A 5 years of the initial diagnosis she developed on and off motor fluctuations, which responded initially to the optimized clinical treatment. About 2 years after, she was still experiencing frequent on and off periods associated with dyskinesias. This patient was assessed again by a movement disorders neurologist and was referred to the DBS surgical counseling. She underwent a full subthalamic nucleus DBS implant procedure in November 2018. In January 2019, during her recovery, she fell and sustained a minor head trauma, with abrasions on the lateral incision located close to the connector’s site without wound dehiscence. In March 2019, an erosion of the connectors site [
DISCUSSION
DBS is an effective and established treatment for movement disorders.[
Several techniques were described in the literature for the management of scalp erosions related to brain stimulation implants. The most commonly used treatments are debridement and simple skin closure followed by antibiotics, as well as removal and/or replacement of parts of the system.[
In a prospective analysis of 144 patients who underwent DBS, Constantoyannis et al. reported that straight incisions on the scalp increased the risk of infection compared with curvilinear incisions.[
In another study with 161 DBS patients, 24 had hardware-related complications. Only one patient had system infection, which occurred in the implanted battery (IPG). Skin erosion occurred in two patients (1.24%), one at the IPG site and one at the connector extension in the retromastoid region. For management of retromastoid erosion, the connector was moved medially to a new occipital bone groove. Skin erosion healed over time without removing the leads and extensions.[
Another case was reported by Lanotte et al., in which one patient presented with a small scalp erosion (2 cm2) after a head trauma, and after excluding the presence of infection, surgical reconstruction was performed. With a portable Doppler, the frontal branch of the right temporal artery was located. The skin covering the device was completely excised and the flap was designed and harvested from the right half of the forehead to the innominate fascia. Another skin triangle was excised to allow flap rotation without tunneling. The donor area was grafted with the skin from the retroauricular region. Three months later, the flap was healthy and without signs of infection.[
To prevent hardware erosion, Barrett et al. used an acellular dermal matrix that was surgically placed under the skin of 20 patients who had imminent signs of erosion. None of the patients treated with this technique required new hardware revision or removal surgery.[
CONCLUSION
There is no standardized technique for managing scalp erosions in the literature. All methods described for managing this type of complication are valid and have satisfactory results. Many high-volume DBS centers have their own experience and way of resolving hardware complications and especially skin erosions. Despite the lack of a unique or single surgical technique suitable for all cases of skin complications, the most important fact is that each case will probably have a more suitable and personalized treatment option. Surgical flaps demonstrated to be efficient resolving skin erosions and also skin retractions as well. For some cases creating grooves on the skull bone can also be performed to bury the connections. In this particular case report, we demonstrated that mobilizing the connectors anteriorly to be overlapped and wrapped by the temporal muscle was effective in this case without the use of a skin flap. Also using VP seems to be efficient and prevents infection.
Statement of ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Abode-Iyamah KO, Chiang HY, Woodroffe RW, Park B, Jareczek FJ, Nagahama Y. Deep brain stimulation hardware-related infections: 10-year experience at a single institution. J Neurosurg. 2018. 129: 1-10
2. Aum DJ, Tierney TS. Deep brain stimulation: Foundations and future trends. Front Biosci (Landmark Ed). 2018. 23: 162-82
3. Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: Systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015. 83: 816-23
4. Barrett TF, Rasouli JJ, Taub P, Kopell BH. Technical note: Preemptive surgical revision of impending deep brain stimulation hardware erosion. World Neurosurg. 2018. 111: 41-6
5. Beitz JM. Parkinson’s disease: A review. Front Biosci. 2014. 6: 65-74
6. Bernstein JE, Kashyap S, Ray K, Ananda A. Infections in deep brain stimulator surgery. Cureus. 2019. 11: e5440
7. Boviatsis EJ, Stavrinou LC, Themistocleous M, Kouyialis AT, Sakas DE. Surgical and hardware complications of deep brain stimulation. A seven-year experience and review of the literature. Acta Neurochir (Wien). 2010. 152: 2053-62
8. Constantoyannis C, Berk C, Honey CR, Mendez I, Brownstone RM. Reducing hardware-related complications of deep brain stimulation. Can J Neurol Sci. 2005. 32: 194-200
9. Crowell JL, Shah BB. Surgery for dystonia and tremor. Curr Neurol Neurosci Rep. 2016. 16: 22
10. Doshi PK. Long-term surgical and hardware-related complications of deep brain stimulation. Stereotact Funct Neurosurg. 2011. 89: 89-95
11. Doshi PK, Rai N, Das D.editors. Surgical and hardware complications of deep brain stimulation-a single surgeon experience of 519 cases over 20 years. Neuromodulation. 2021. p.
12. Franceschini PH, Bhargava D, Eldridge PR. Outcomes following deep brain stimulation for Parkinson’s disease: A single centre experience. Arq Bras Neurocir Braz Neurosurg. 2018. 37:
13. Gomez R, Hontanilla B. The reconstructive management of hardware-related scalp erosion in deep brain stimulation for Parkinson disease. Ann Plast Surg. 2014. 73: 291-4
14. Hamani C, Lozano AM. Hardware-related complications of deep brain stimulation: A review of the published literature. Stereotact Funct Neurosurg. 2006. 84: 248-51
15. Hu X, Jiang X, Zhou X, Liang J, Wang L, Cao Y. Avoidance and management of surgical and hardware-related complications of deep brain stimulation. Stereotact Funct Neurosurg. 2010. 88: 296-303
16. Jitkritsadakul O, Bhidayasiri R, Kalia SK, Hodaie M, Lozano AM, Fasano A. Systematic review of hardware-related complications of deep brain stimulation: Do new indications pose an increased risk?. Brain Stimul. 2017. 10: 967-76
17. Lanotte M, Verna G, Panciani PP, Taveggia A, Zibetti M, Lopiano L. Management of skin erosion following deep brain stimulation. Neurosurg Rev. 2009. 32: 111-4
18. Lozano AM, Lipsman N, Bergman H, Brown P, Chabardes S, Chang JW. Deep brain stimulation: Current challenges and future directions. Nat Rev Neurol. 2019. 15: 148-60
19. McKinnon C, Gros P, Lee DJ, Hamani C, Lozano AM, Kalia LV. Deep brain stimulation: Potential for neuroprotection. Ann Clin Transl Neurol. 2019. 6: 174-85
20. Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. 2017. 32: 1264-310
21. Patel DM, Walker HC, Brooks R, Omar N, Ditty B, Guthrie BL. Adverse events associated with deep brain stimulation for movement disorders: Analysis of 510 consecutive cases. Neurosurgery. 2015. 11: 190-9
22. Rasouli JJ, Kopell BH. The adjunctive use of vancomycin powder appears safe and may reduce the incidence of surgical-site infections after deep brain stimulation surgery. World Neurosurg. 2016. 95: 9-13
23. Sixel-Doring F, Trenkwalder C, Kappus C, Hellwig D. Skin complications in deep brain stimulation for Parkinson’s disease: Frequency, time course, and risk factors. Acta Neurochir (Wien). 2010. 152: 195-200
24. Sorar M, Hanalioglu S, Kocer B, Eser MT, Comoglu SS, Kertmen H. Experience reduces surgical and hardware-related complications of deep brain stimulation surgery: A single-center study of 181 patients operated in six years. Parkinsons Dis. 2018. 2018: 3056018
25. Spiotta AM, Bain MD, Deogaonkar M, Boulis NM, Rezai AR, Hammert W. Methods of scalp revision for deep brain stimulator hardware: Case report. Neurosurgery. 2008. 62: 249-50
26. Staudt MD, Pourtaheri N, Lakin GE, Soltanian HT, Miller JP. Surgical management of deep brain stimulator scalp erosion without hardware removal. Stereotact Funct Neurosurg. 2017. 95: 385-91
27. Sui Y, Tian Y, Ko WK, Wang Z, Jia F, Horn A. Deep brain stimulation initiative: Toward innovative technology, new disease indications, and approaches to current and future clinical challenges in neuromodulation therapy. Front Neurol. 2021. 11: 597451
28. Zhou R, Ma Y, Liu W, Miao S, Zhang Y. Long-term effect of modified incision to prevent related complications in deep brain stimulation. World Neurosurg. 2018. 117: 280-3