- Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Otorhinolaryngology, Indus Hospital, Sahibzada Ajit Singh Nagar, Punjab, India.
- Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
- Department of Neuroradiodiagnosis, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Correspondence Address:
Manjul Tripathi, Department of Neurosurgery, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
DOI:10.25259/SNI_472_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Ninad Ramesh Patil1, Manjul Tripathi1, Kshitij Charaya2, Archana Angrup3, Chirag Ahuja4, Sandeep Mohindra1. Skull base osteomyelitis by Pandoraea apista: An unusual pathogen at unusual location – A case report. 06-Sep-2021;12:447
How to cite this URL: Ninad Ramesh Patil1, Manjul Tripathi1, Kshitij Charaya2, Archana Angrup3, Chirag Ahuja4, Sandeep Mohindra1. Skull base osteomyelitis by Pandoraea apista: An unusual pathogen at unusual location – A case report. 06-Sep-2021;12:447. Available from: https://surgicalneurologyint.com/surgicalint-articles/11094/
Abstract
Background: Pandoraea apista is predominantly recovered from the respiratory tract of patients with cystic fibrosis (CF). Authors report first case of central nervous system infection by P. apista in the form of skull base osteomyelitis.
Case Description: A 67-year-old male presented with complaints of earache and hearing deficit for few months. The radiology was suggestive of skull base osteomyelitis and polypoidal soft tissue extending from the middle cranial fossa to the infratemporal fossa. The sample from the targeted area revealed P. apista on matrix-assisted laser desorption ionization-time-of-flight mass spectrometry. With adequate antibiotic therapy, there was clinicoradiologic improvement. P. apista is an infection exclusively seen in pulmonary infection in patients with CF. We identified its intracranial involvement in a patient for the 1st time in the literature. The serendipitous diagnosis needs evaluation on specific PCR and matrix-assisted laser desorption spectrometry. The treatment with antibiotics provides a definite cure.
Conclusion: We report a rare opportunistic infection with central nervous system involvement which can be cured by accurate diagnosis and appropriate antibiotic treatment.
Keywords: Cystic fibrosis, Infection, Matrix-assisted laser desorption ionization-time-of-flight mass spectrometry, Pandoraea apista, PCR
INTRODUCTION
Pandoraea genus is a relatively new genus first described by Coenye et al. in 2000. The name Pandoraea was given to this genus to refer to Pandora’s box (from Greek mythology which was considered as the origin of diseases to the mankind), because of surprising diversity amongst the members of this genus.[
CASE REPORT
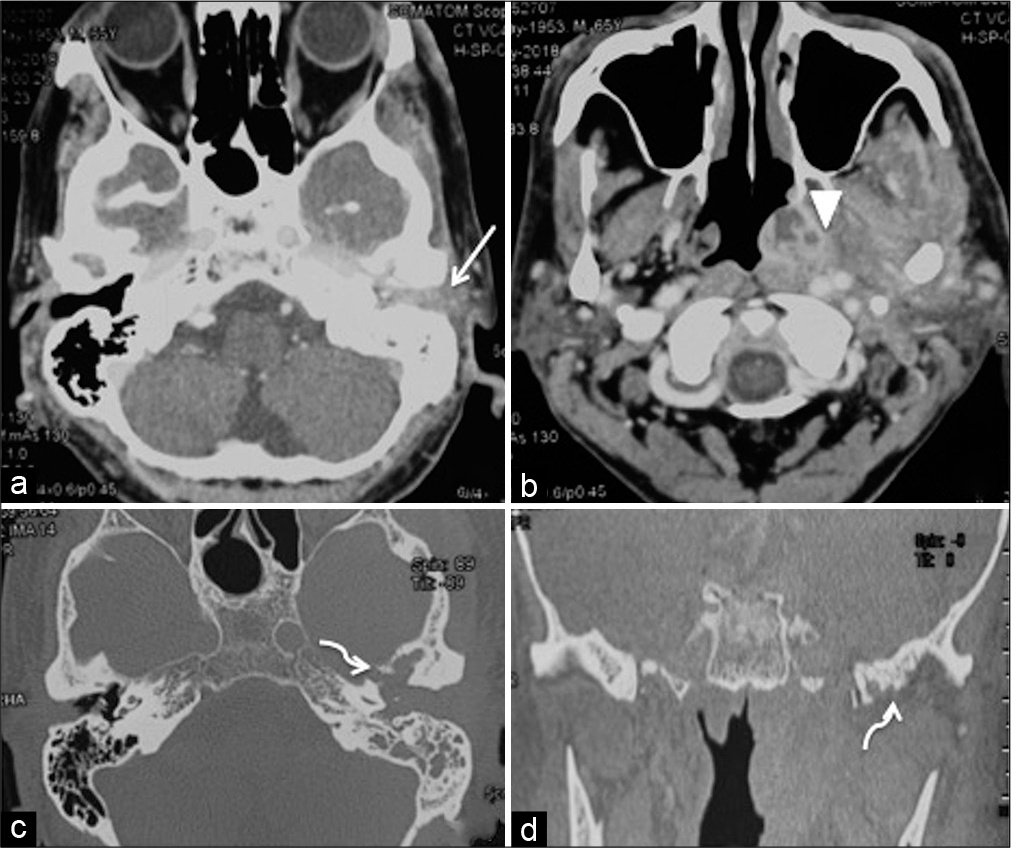
A 67-year-old male presented with complaint of severe pain in the left ear along with decreased hearing for few months. Examination revealed a bulge in the external auditory canal making the tympanic membrane visualization impossible. The patient had nonserviceable conductive and sensorineural hearing loss in the left ear. His systemic and other neurological examination was nonremarkable. CT scan revealed poorly defined heterogeneous enhancing soft-tissue mass involving external auditory canal, pterygoid space, masticator space, nasopharynx, and infratemporal fossa. CT scan of skull base revealed polypoidal soft tissue occluding the left external auditory canal [
Figure 1:
Contrast-enhanced CT scan axial soft-tissue window (a and b), axial (c) and coronal bone window (d) sections revealing polypoidal soft tissue occluding the external auditory meatus (arrow) with increased bulk and heterogeneous enhancement (arrowhead) of the nasopharynx, base of skull, and masticator muscles signifying inflammatory soft tissue. Bone window sections showing erosive changes in the bones of the skull base (curved arrows) signifying osteomyelitis.
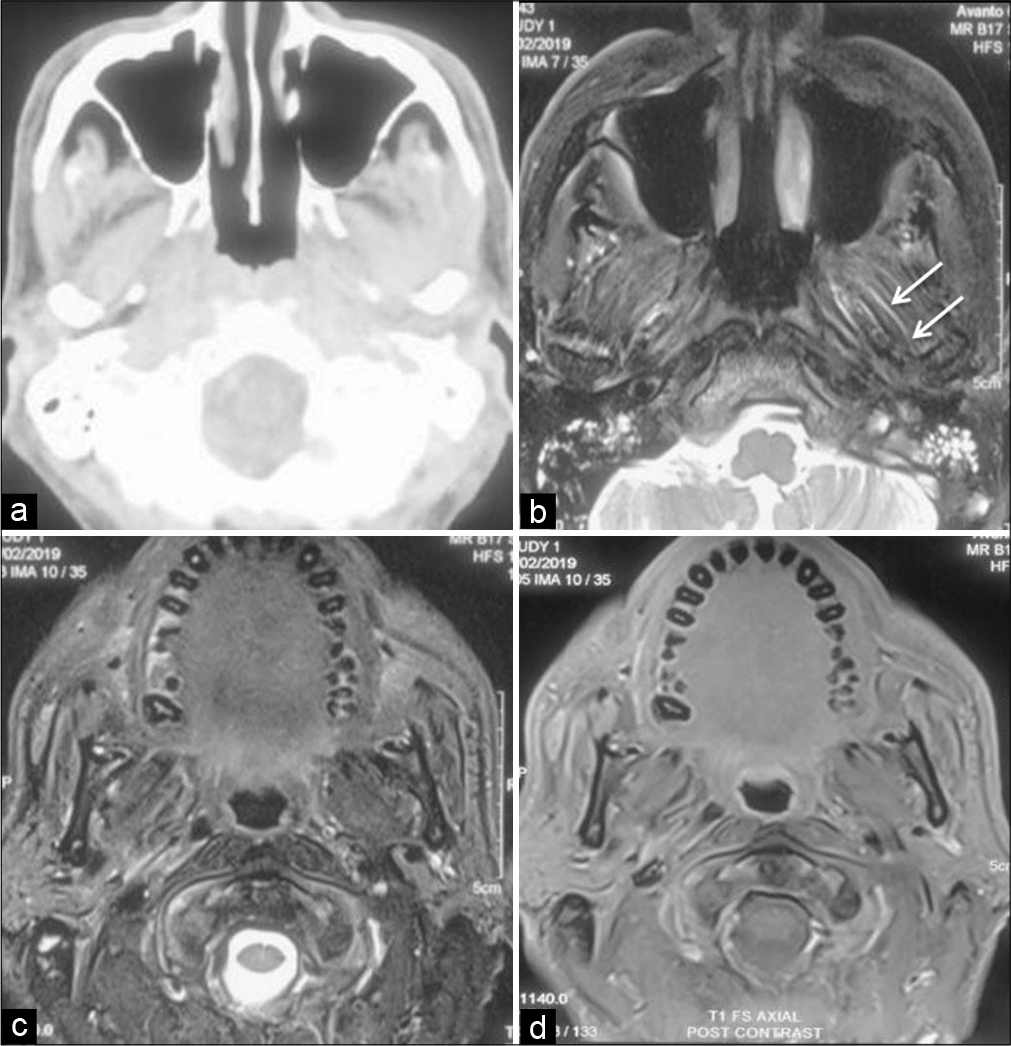
The positron emission tomography scan neither revealed any other pathology nor showed any metabolic activity. Considering clinical and radiologic considerations, the possibilities of skull base osteomyelitis or a neoplastic lesion were considered. Biopsy from nasopharynx was inconclusive while CT-guided FNAC from skull base was sent for bacterial culture. The sample was inoculated on blood agar and MacConkey agar and incubated at 37°C overnight under aerobic conditions as a part of routine processing for aerobic isolates. The culture revealed monomicrobial growth of a nonlactose fermenting colony on MacConkey agar which was identified as Pandoraea apista on matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF, MS, Bruker Daltonik GmbH, Germany) using the MALDI BioTyper database. The patient was then kept on ciprofloxacin 750 mg twice a day for 1-month duration. This led to relief from pain and subsequent imaging showed gradual resolution of lesion over many moths with disappearance of the bulge of the ear canal. Follow-up CT and MRI performed after 1 year showed near-complete resolution of the lesion [
Figure 2:
Axial CECT (a) showing near complete resolution of the inflammation in the base of skull region and masticator space. MRI (axial T2 FS-[b and c] and contrast-enhanced T1-[d]) of the corresponding area confirms these findings with minimal inflammation along the muscle fibers (arrows) with no contrast enhancement.
DISCUSSION
At present, Pandoraea genus consists of 10 exclusive human clinical species. These include P. apista, Pandoraea pulmonicola, Pandoraea sputorum, Pandoraea anapnoica, Pandoraea anhela, Pandoraea bronchicola, Pandoraea captiosa, Pandoraea morbifera, Pandoraea nosoerga, and Pandoraea pneumonica. Nonclinical species are mainly isolated from soil, water, animal feces, and powdered milk.[
Pathogenesis and virulence factor
Human Pandoraea species are primarily isolated from respiratory secretions of patients of cystic fibrosis (CF).
Seldom, from non-CF patients, has also been reported [
All Pandoraea species stimulate release of pro-inflammatory cytokines, particularly IL-6 and IL-8. Upregulation of these two interleukins is the primary virulence factor which is largely responsible for the chronic lung inflammation. Chronic colonization of Pandoraea further leads to in vivo point mutations and strain evolution overtime.[
In CF, lung infection is polymicrobial in nature with Pseudomonas aeruginosa as the most commonly colonizing pathogen. P. aeruginosa and Burkholderia multivorans have growth suppressing effects on Pandoraea species, that is, why Pandoraea is more virulent in the absence of co-colonization with these pathogens and is more likely to cause bacteremia in non-CF patients than CF patients. Therefore, younger patients who had higher pathogen diversity showed lower incidence of permanent lung damage than older patients.[
Diagnosis
Routine diagnostic tests are not sufficient for the diagnosis of Pandoraea species and molecular studies are required for their identification.[
Recently, the use of MALDI-TOF, MS has shown good results. MALDI-TOF MS designates an organic matrix compound, which helps in the soft desorption and ionization of highly abundant bacterial or fungal proteins using laser energy. Subsequently, accurate identification relies on the availability of well-curated, extensive databases preferably encompassing the full spectrum of microbes encountered in clinical specimens.[
With its use, early identification of the bacteria is possible. The use of MALDI-TOF MS for the routine identification of most microbes has largely replaced conventional biochemical and phenotypic tests in clinical laboratories, since it is 1.45 days faster, less expensive, and does not involve a labor-intensive protocol.[
Antibiotic susceptibility
Multidrug resistance is because of enzyme production and efflux pumps. Oxacillinase-62 plays an important role against imipenem resistance. All show resistance to meropenem, aminoglycosides, quinolones, and most β-lactam antibiotics while they are susceptible to imipenem, tetracycline, and trimethoprim-sulfamethoxazole. Its greatly variable antibiotic susceptibility necessitates accurate diagnosis and antibiotic sensitivity for its treatment.[
There is no report so far for the intracranial invasion of P. apista. Authors present the very first case of this pathogen showing involvement of central nervous system. We are not able to identify the source of this pathogen in the patient. There is no case reported so far from our region. Surprisingly, the patient has no host factor to suggest any predicament or susceptibility for this extremely rare pathogen. The identification of the pathogen in our case is a serendipity and we could manage the case only after going through the available literature.
CONCLUSION
Pandoraea is responsible for opportunistic pulmonary infections predominantly affecting CF patients. Although it can also affect non-CF patients, in whom it may be more virulent because of absence of polymicrobial environment. It requires molecular analysis for its accurate diagnosis. Because of its multidrug resistance, antibiotic sensitivity is necessary for the treatment. This pathogen may present with primary involvement of central nervous system and a high index of suspicion is necessary for its diagnosis and management.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Publication of this article was made possible by the James I. and Carolyn R. Ausman Educational Foundation.
Conflicts of interest
There are no conflicts of interest.
References
1. Almuzara M, Barberis C, Traglia G, Famiglietti A, Ramirez MS, Vay C. Evaluation of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry for species identification of non-fermenting Gram-negative bacilli. J Microbiol Methods. 2015. 112: 24-7
2. Caraher E, Collins J, Herbert G, Murphy PG, Gallagher CG, Crowe MJ. Evaluation of in vitro virulence characteristics of the genus Pandoraea in lung epithelial cells. J Med Microbiol. 2008. 57: 15-20
3. Coenye T, Falsen E, Hoste B, Ohlén M, Goris J, Govan JR. Description of Pandoraea gen. nov. with Pandoraea apista sp. nov., Pandoraea pulmonicola sp. nov., Pandoraea pnomenusa sp. nov., Pandoraea sputorum sp. nov. and Pandoraea norimbergensis comb. nov. Int J Syst Evol Microbiol. 2000. 50: 887-99
4. Coenye T, Liu L, Vandamme P, LiPuma JJ. Identification of Pandoraea species by 16S ribosomal DNA-based PCR assays. J Clin Microbiol. 2001. 39: 4452-5
5. Costello A, Reen FJ, O’Gara F, Callaghan M, McClean S. Inhibition of co-colonizing cystic fibrosis-associated pathogens by Pseudomonas aeruginosa and Burkholderia multivorans. Microbiology. 2014. 160: 1474-87
6. Daneshvar MI, Hollis DG, Steigerwalt AG, Whitney AM, Spangler L, Douglas MP. Assignment of CDC weak oxidizer group 2 (WO-2) to the genus Pandoraea and characterization of three new Pandoraea genomospecies. J Clin Microbiol. 2001. 39: 1819-26
7. Falces-Romero I, Gutiérrez-Arroyo A, Romero-Gómez MP. Bacteriemia asociada a catéter por Pandoraea pnomenusa en un paciente pediátrico con leucemia aguda linfoblástica. Med Clín. 2016. 147: 132
8. Gao J, Wei Q, Shao H, Wu H. A case report of Pandoraea sp isolated from neonatal blood culture. Chin J Microbes Infect. 2018. 13: 368-72
9. Greninger AL, Streithorst J, Golden JA, Chiu CY, Miller S. Complete genome sequence of sequential Pandoraea apista isolates from the same cystic fibrosis patient supports a model of chronic colonization with in vivo strain evolution over time. Diagn Microbiol Infect Dis. 2017. 87: 1-6
10. Lin C, Luo N, Xu Q, Zhang J, Cai M, Zheng G. Pneumonia due to Pandoraea apista after evacuation of traumatic intracranial hematomas: A case report and literature review. BMC Infect Dis. 2019. 19: 869
11. Monzón T, Valga F, Reichert J, López C. Hemodialysis catheter colonised by Pandoraea spotorum. Nefrología. 2018. 38: 662-4
12. Peeters C, de Canck E, Cnockaert M, de Brandt E, Snauwaert C, Verheyde B. Comparative genomics of Pandoraea a genus enriched in xenobiotic biodegradation and metabolism. Front Microbiol. 2019. 10: 2556
13. Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front Microbiol. 2015. 6: 791
14. Stryjewski ME, LiPuma JJ, Messier RH, Reller LB, Alexander BD. Sepsis, multiple organ failure, and death due to Pandoraea pnomenusa infection after lung transplantation. J Clin Microbiol. 2003. 41: 2255-7
15. Xiao X, Tian H, Cheng X, Li G, Zhou J, Peng Z. Pandoraea sputorum bacteremia in a patient who had undergone allogeneic liver transplantation plus immunosuppressive therapy: A case report. Infect Drug Resist. 2019. 12: 3359-64
16. Yong D, Tee KK, Yin WF, Chan KG. Characterization and comparative overview of complete sequences of the first plasmids of Pandoraea across clinical and non-clinical strains. Front Microbiol. 2016. 7: 1606