- Department of Neurosurgery, Riuniti Hospital, Foggia, Italy
- Department of Neurosurgery, University of Foggia, Foggia, Italy
- Department of Pathology, “Riuniti” Hospital, Foggia, Italy,
- Department of Neurosurgery, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany,
- Department of Neurosurgery, Division of Neurosurgery, San Pio Hospital, Benevento, Italy
- Department of Medical Oncology and Bimolecular Therapy, “Riuniti” Hospital, Foggia,, Italy
- Department of Radiological, Oncological and Pathological Sciences, La Sapienza University, Roma, Lazio, Italy
- Department of Anatomical, Histological, Forensic Medicine and Orthopedic sciences, La Sapienza University, Roma, Lazio, Italy.
Correspondence Address:
Augusto Leone, Department of Neurosurgery, Städtisches Klinikum Karlsruhe, Karlsruhe, Germany.
DOI:10.25259/SNI_722_2022
Copyright: © 2022 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Antonio Colamaria1, Francesco Carbone2, Matteo Sacco1, Fabrizio Corsi3, Augusto Leone4, Giovanni Parbonetti5, Matteo de Notaris5, Nicola Pio Fochi2, Matteo Landriscina6, Giulia Coppola7, Elena de Santis8, Guido Giordano6. Solitary fibrous tumor/hemangiopericytoma of the cervical spine: A systematic review of the literature with an illustrative case. 18-Nov-2022;13:532
How to cite this URL: Antonio Colamaria1, Francesco Carbone2, Matteo Sacco1, Fabrizio Corsi3, Augusto Leone4, Giovanni Parbonetti5, Matteo de Notaris5, Nicola Pio Fochi2, Matteo Landriscina6, Giulia Coppola7, Elena de Santis8, Guido Giordano6. Solitary fibrous tumor/hemangiopericytoma of the cervical spine: A systematic review of the literature with an illustrative case. 18-Nov-2022;13:532. Available from: https://surgicalneurologyint.com/surgicalint-articles/12014/
Abstract
Background: In the WHO 2016 classification of central nervous system tumors, solitary fibrous tumors (SFT) and hemangiopericytomas (HPC) were considered part of the same category given a shared mutation. Nevertheless, since the new 2021 WHO classification, the term “hemangiopericytoma” has been retired, and SFT is considered an independent pathological entity.
Methods: We reviewed the literature following preferred reporting items for systematic reviews and meta-analyses guidelines focusing on the treatment options and prognosis of patients with cervical SFT. We also present a 68-year-old female with spinal intradural extramedullary SFT complicated by diffuse extension into paravertebral tissues and muscles.
Results: We found 38 cervical SFT in the literature. Patients averaged 47.3 years of age and 47.4% were female. Typically, these lesions spanned two spinal levels resulting in cord compression and most frequently exhibited benign features (i.e., diagnosed as Grade I SFTs). Interestingly, two patients exhibited distant metastases and had initial pathology consistent with grade II SFT.
Conclusion: SFT of the cervical spine is rare and its management varies according to the histological grade and the clinical behavior, generally warranting surgical excision and adjuvant radiation therapy and/or systemic chemotherapy.
Keywords: Adjuvant chemotherapy, Cervical spine, Hemangiopericytoma, Solitary fibrous tumor, Spinal tumor
INTRODUCTION
Solitary fibrous tumor/hemangiopericytoma (SFT/HPC) are since the new 2021 WHO classification of central nervous system tumors the same entity and account for 1.9–4% of intracranial neoplasms, with few being found in the spine.[
MATERIALS AND METHODS
A qualitative review of the literature was performed in compliance with the updated preferred reporting items for systematic reviews and meta-analyses guidelines using the electronic database MEDLINE/PubMed.[
CASE DESCRIPTION
Clinical and radiological presentation
A 68-year-old female presented with a 6-month history of poorly localized cervicalgia, bilateral brachialgia, and hyposthenia of the upper extremities accompanied by progressive 2/5 paresis of the upper extremities with decreased sensation to touch and burning paresthesia in both arms (i.e., increased in the left hand). Proprioception and vibration were intact.
Radiological assessment
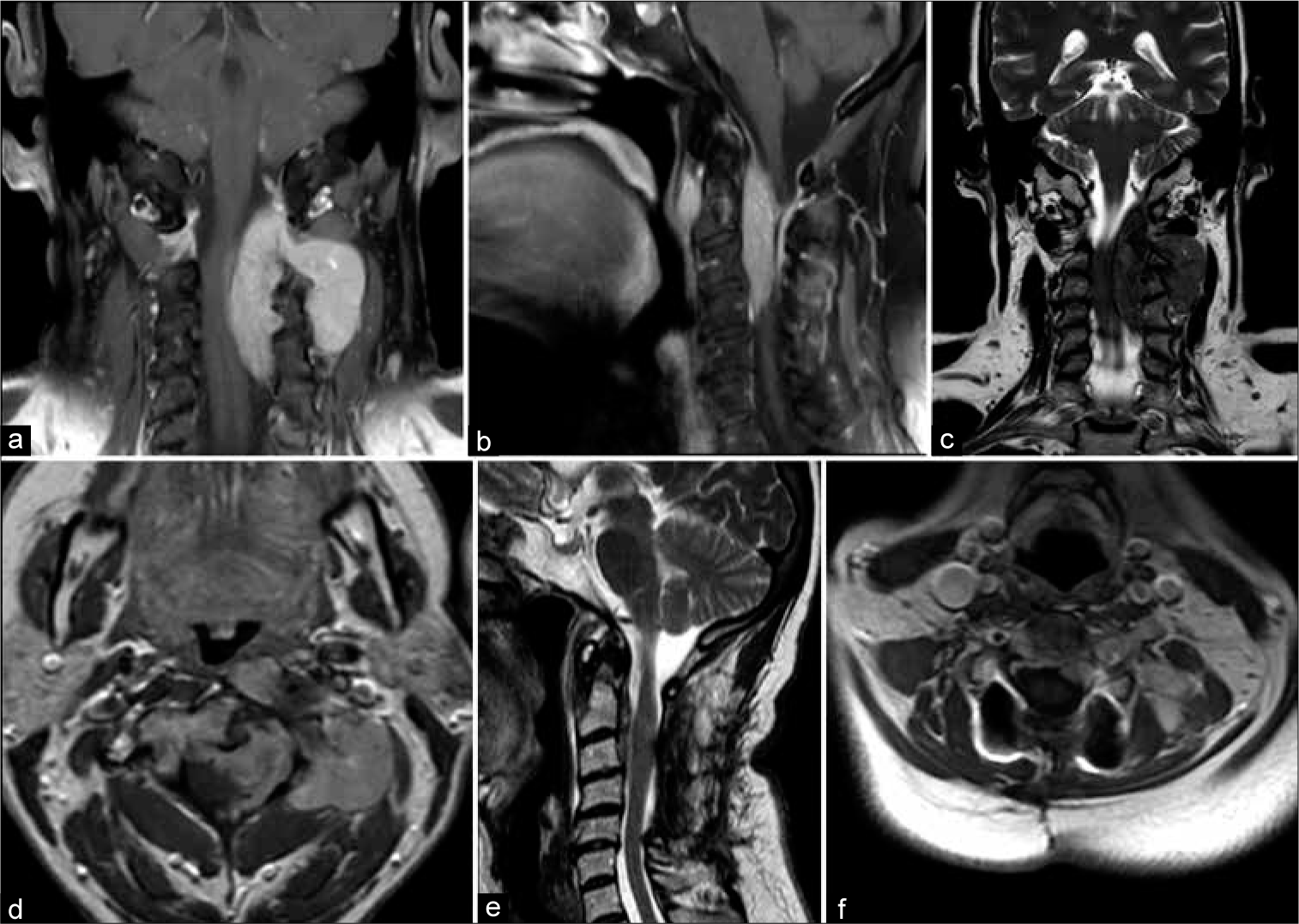
The entire spine was evaluated with magnetic resonance imaging (MRI). It revealed a cervical 8 cm, poorly circumscribed, intra-extradural tumor located between C0 and C5 level with epidural extension, and cord compression [
Figure 2:
(a) Coronal and (b) sagittal T1-weighted images demonstrating the diffusely infiltrating hyperintense tumor causing marked compression on the left and anterior spinal cord surfaces. Of notice the extraspinal extension through the left third and fourth foramina and the anterior component of the lesion located anteriorly to the third and fourth vertebral bodies. (c) Sagittal T2-weighted scan showing the inhomogeneous hypointensity of the lesion. (d) The paraspinal and the intra-extradural components of the tumor exhibited homogeneous gadolinium enhancement. (e) Postoperative T2-weighted image documented successful decompression of the spinal cord and debulking of the intraspinal component of the tumor. (f) Axial T1-weighted image after the administration of contrast showing considerable reduction of the paraspinal component of the solitary fibrous tumors/hemangiopericytomas following the four cycles of chemotherapy.
Surgical procedure
The patient agreed to a C2–C5 laminectomy with bilateral lateral mass resection and instrumented fusion to achieve a subtotal tumor resection (STR) through a posterior-only approach. The paramedian dural incision revealed a white-gray-colored mass compressing the left anterolateral surface of the spinal cord shifting tumor to the right. Tumor was debulked/decompressed, as confirmed by the postoperative MRI [
Postoperative course and pathology
Postoperatively, the patient improved demonstrating reduced muscular weakness and brachialgia; she was discharged on the 4th postoperative day and referred to a rehabilitation center. She refused a potential secondary anterior tumor resection due to the risk of increased morbidity.
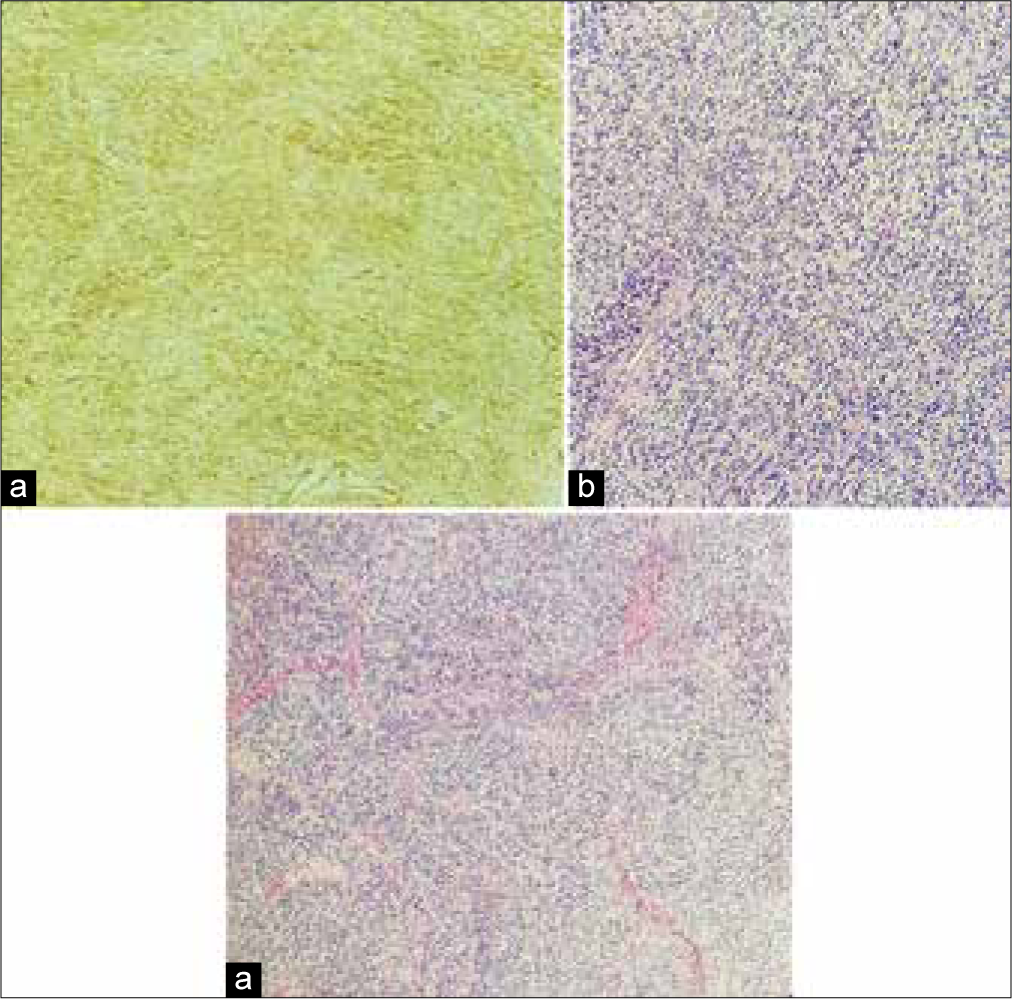
The permanent histological sections documented a pattern consistent with the diagnosis of SFT [
Figure 3:
Histological images showing (a) a moderately cellular tumor exhibiting ovoidal and spindle cells arranged in small fascicles and embedded in a collagenous stroma (Hematoxylin eosin). (b) Malignant cells did not show neither signs of cytologic atypia nor necrosis and present a low mitotic count (Hematoxylin eosin). (c) Immunohistochemistry shows strong and diffuse positivity for CD34, a pattern consistent with the diagnosis of solitary fibrous tumors.
Adjuvant therapy and follow-up
A month after surgical debulking, the patient received radiation therapy (24 Gray) but no further surgery. She also agreed to undergo adjuvant chemotherapy with four cycles of Epirubicin. An MRI following the completion of chemotherapy revealed a sensible reduction of the residual extraspinal component of tumor-infiltrating the paraspinal muscles [
REVIEW OF THE LITERATURE
Patient characteristics
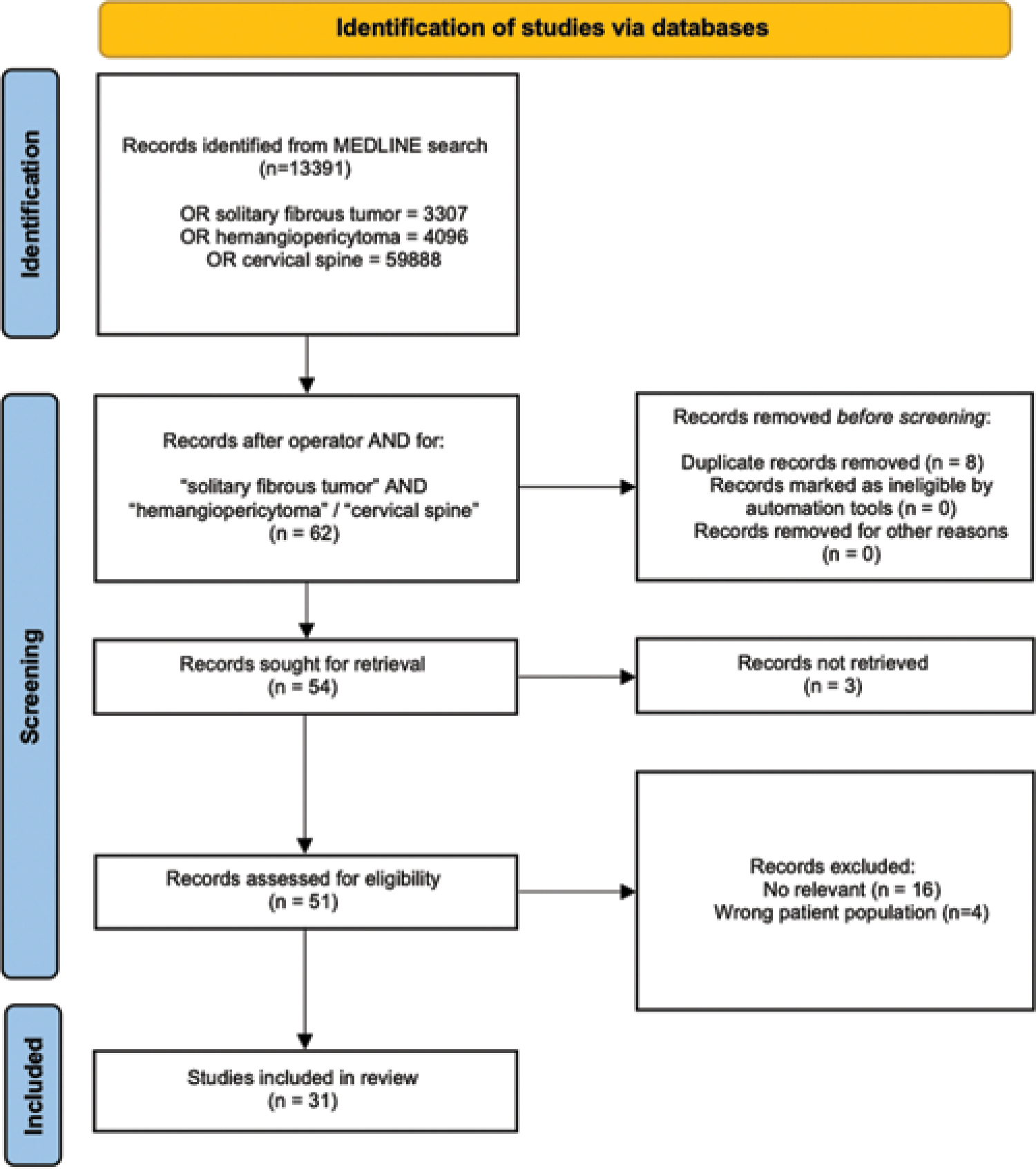
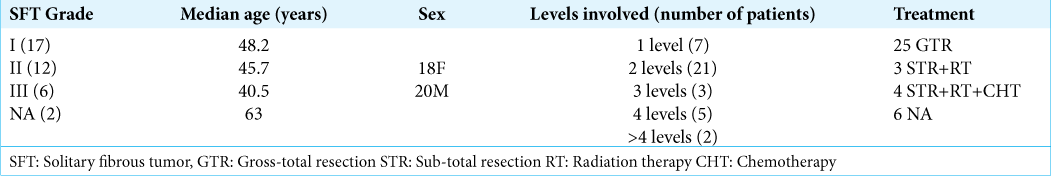
We evaluated 31 articles concerning 38 cases (including this one) of cervical SFT/HPC: 29 case reports and two case series (i.e., largest cohort of five patients). Patients averaged 48.2 years of age for Grade I, 45.7 years old with Grade II SFT, and 40.5 for Grade III HPC lesions. Seven tumors (18.4%) involved one level, while 21 (55.3%) occupied two levels of the spinal cord, three tumors (7.9%) extended to three levels, 5 (10.5%) involved four levels, and two tumors (5.3%) extended for >4 levels [
Tumor characteristics and management
In 25 cases (65%), gross-total resection (GTR) of the tumor was performed as the sole treatment, whereas in three cases (7.9%), surgical treatment was followed by radiation therapy and in four patients (10.5%), adjuvant chemotherapy was also deployed.
DISCUSSION
Since 2021, the WHO classified SFT into three grades: a Grade I that corresponds to the highly collagenous, relatively low cellularity with typical spindle cells previously diagnosed as SFT; a Grade II, frequently more cellular and exhibiting less collagenous component with “staghorn” vasculature that was previously designated as HPC; and a Grade III that most often corresponds to what was termed anaplastic HPC.[
In their systematic review, Giordan et al.[
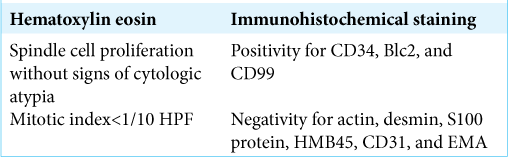
Microscopic examination of this neoplasm demonstrates a patternless architecture composed of spindle-shaped cells with different grades of atypia disposed between thick bands of collagen. Confirmation of the diagnosis is made with immunohistochemical staining, with abnormal cells exhibiting positivity for CD34, CD99, Bcl2, vimentine, and no signs of reaction for S100 protein and EMA.[
RADIOLOGICAL DIAGNOSIS
Frequently encountered radiological features of SFT include hypo/isointensity on T1-weighted images with a heterogeneous enhancement after the administration of contrast.[
Prognosis
A recent retrospective study Sung et al.[
CONCLUSION
SFT of the cervical spine is rare and relatively benign tumors, whose preferable treatment lays within a combination of surgical tumor resection, radiation therapy, and adjuvant chemotherapy.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: A lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996. 106: 217-24
2. Giordan E, Marton E, Wennberg AM, Guerriero A, Canova G. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev. 2021. 44: 1299-312
3. Gubian A, Ganau M, Cebula H, Todeschi J, Scibilia A, Noel G. Intracranial solitary fibrous tumors: A heterogeneous entity with an uncertain clinical behavior. World Neurosurg. 2019. 126: e48-56
4. Jia Q, Zhou Z, Zhang D, Yang J, Liu C, Wang T. 2018 Surgical management of spinal solitary fibrous tumor/ hemangiopericytoma: A case series of 20 patients. Eur Spine J. 2018. 27: 891-901
5. Kurtkaya O, Elmaci I, Sav A, Pamir MN. Spinal solitary fibrous tumor: Seventh reported case and review of the literature. Spinal Cord. 2001. 39: 57-60
6. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol. 2021. 23: 1231-51
7. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021. 372: n71
8. Sung KS, Moon JH, Kim EH, Kang SG, Kim SH, Suh CO. Solitary fibrous tumor/hemangiopericytoma: Treatment results based on the 2016 WHO classification. J Neurosurg. 2018. p. 1-8