- Department of Neurosurgery, Emory University School of Medicine, Atlanta, Georgia, United States.
Correspondence Address:
Zachary Bernstein, Emory University School of Medicine, Atlanta, Georgia, United States.
DOI:10.25259/SNI_77_2023
Copyright: © 2023 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Zachary Bernstein, David Cory Adamson. Solitary metastasis to the skull as the first sign of hepatocellular carcinoma in a patient in long-term remission. 21-Jul-2023;14:252
How to cite this URL: Zachary Bernstein, David Cory Adamson. Solitary metastasis to the skull as the first sign of hepatocellular carcinoma in a patient in long-term remission. 21-Jul-2023;14:252. Available from: https://surgicalneurologyint.com/surgicalint-articles/12453/
Abstract
Background: Hepatocellular carcinoma (HCC) is a common malignant tumor with a 5-year survival rate of 10%, presenting with extrahepatic metastases in 15–17% of patients. HCC-bone metastases represent approximately one-quarter of all HCC metastases, most frequently in the spine, pelvis, ribs, or femur. HCC-skull metastases, however, make up 0.4–1.6% of all HCC- bone metastases. Furthermore, solitary HCC-skull metastasis without known active primary HCC is an unusual presentation warranting further review and consideration.
Case Description: Here, the authors report a unique case of a solitary HCC-skull metastasis in a patient without known active cancer but in long-term remission for HCC. The patient is a 69-year-old male with past HCC who presented with a nontender skull mass. A computed tomography scan showed a heterogeneously enhancing mass centered in the high left parietal bone with intracranial extension. There was a noted mass effect on the left posterior frontoparietal region without worrisome midline shift. Pathology ultimately revealed the mass to be metastatic HCC. To aid in the understanding and clinical management of this rare presentation, we reviewed the literature regarding clinical presentation, radiological features, pathology, and outcome.
Conclusion: Ultimately, early detection of the primary source of cancer is pivotal to successful treatment and prognosis, and skull lesions such as these must include HCC in the differential diagnosis.
Keywords: Hepatocellular carcinoma, Liver metastasis, Neurosurgery, Skull metastasis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common malignant tumor and has a 5-year survival rate of 10%.[
Jiang et al. described 59 cases of HCC-skull metastasis within the literature in 2014, of which only 14 cases presented as a solitary skull lesion.[
MATERIALS AND METHODS
For the case report, the patient was identified by the senior author when presenting to the clinic. No personal identifiers are disclosed and the write-up of this manuscript is in accordance with the corresponding institution’s IRB and ethical guidelines. The patient’s clinical history, presentation, and management are discussed.
For the review of the literature, PubMed was used. Search terms included: “Hepatocellular carcinoma,” “HCC metastasis,” “HCC skull metastasis,” and “skull metastasis.” The resulting search results were manually filtered by authors to include articles with a title or abstract that only featured both HCC and solitary skull metastasis. Six articles were found and are highlighted.
CASE DESCRIPTION
The patient is a 69-year-old male with chronic obstructive pulmonary disease, glaucoma, cirrhosis, and past HCC who began to grow a nontender skull mass in November 2021. He did not seek care until June 2022, when he presented with focal headache at the site of the skull mass. There were no relevant findings from the general physical examination or neurological examination. The patient was neurologically intact with a soft scalp mass that was nontender with no overlying skin stigmata. The mass measured approximately 6 × 6 cm and protruded to 3 cm above the scalp surface. The scalp and hair looked completely normal except being slightly stretched over the underlying tumor. The patient did not have any associated lymphadenopathy.
The patient’s oncology history is significant for HCC that was diagnosed in 2015. This was found on screening abdominal ultrasound showing a single hypoechoic lesion at the posterior right lobe of the liver measuring 2.4 × 2.1 × 2.2 cm with a 1.9 cm cyst in the inferior segment. After diagnostic biopsy, the patient underwent transarterial chemoembolization therapy to both hepatic lesions complicated by abdominal pain, constipation, nausea, and vomiting for 7 days. Since this treatment in 2015, the patient has had no recurrence of HCC or any other liver masses or cancer.
The patient declined a head magnetic resonance imaging (MRI). A computed tomography (CT) scan showed a heterogeneously enhancing mass centered in the high left parietal bone that measured approximately 5.0 × 4.5 × 4.6 cm with intracranial extension, but inconclusive on whether the mass was dural, osseous, or metastatic in origin [
Figure 1:
(a) Coronal noncontrast computed tomography (CT) radiograph. (b) Sagittal noncontrast CT radiograph. (c) Axial noncontrast CT radiograph. Scans show heterogeneously enhancing mass centered in the high left parietal bone that measured approximately 5.0 × 4.5 × 4.6 cm with intracranial extension. There is mass effect upon the high left posterior frontoparietal brain where there may have been a trace amount of vasogenic edema. No abnormal parenchymal brain enhancement is noted. No acute intracranial hemorrhage or extra-axial fluid collections were identified. No areas that are strongly suspicious for acute cortical infarction or contusion were seen.
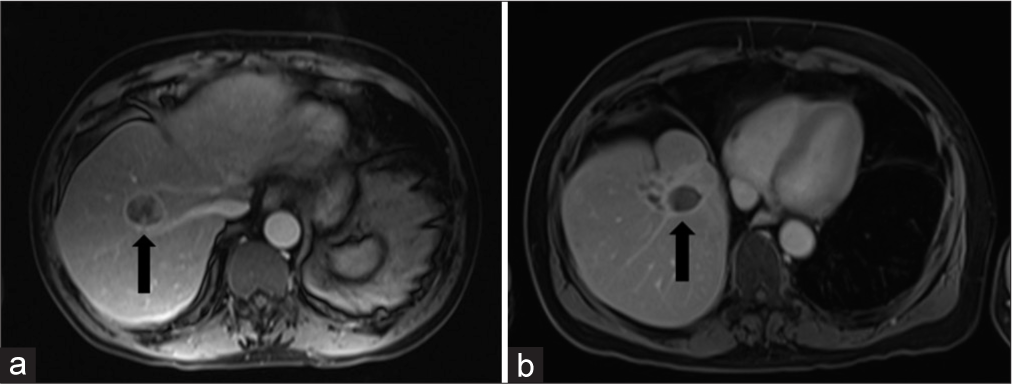
Preoperative evaluation included a CT of chest/abdomen/pelvis showing expected treated hepatic lesions and no new lesions [
Figure 2:
(a) Contrast-enhanced, fat-saturated axial T1 image before treatment. The black arrow points to a rim-enhancing lesion centered in the right hepatic lobe. (b) Contrast-enhanced, fat-saturated axial T1 image after treatment. The black arrow points to the treated lesion in the right hepatic lobe.
The mass was well encapsulated and soft. Inspection after removal revealed a mass centrally necrotic with limited vascularity. Gross total resection was achieved and postoperative chemotherapy and immunotherapy were initiated with Atezolizumab and Bevacizumab. The pathology analysis was consistent with the diagnosis of an intraosseous metastatic HCC-skull tumor without any neural parenchymal involvement. The tumor cells stained positive for cytokeratin AE1, AE3, hepar, and arginase-1 [
Figure 3:
(a) (H&E stain, ×15) Medium power view showing epithelioid tumor cells in cords and trabeculae separated by sinusoids. The underlying tissue has been completely replaced. (b) (H&E stain, ×15) Medium power view showing thickened cords and nests (>2–3 cells thick) of tumor cells and central dilated blood vessel.
Figure 4:
(H&E stain, ×25) High power view showing tumor cells with prominent nucleoli, abundant eosinophilic cytoplasm and well-defined cell borders. Please also note small spindled endothelial cells surrounding the cell nests in so-called endothelial wrapping. Occasional cytoplasmic pigmentation was also identified.
REVIEW
Clinical presentation
Of the five reported cases of solitary HCC-skull metastasis without known primary cancer, the average patient age at time of diagnosis was 53.8 years (range: 40–71 years) [
Radiological features
These reported tumors tended to be large at presentation (mean: 7.5 cm, range: 4 cm, 11 cm). Tumor location varied, but involved the occiput in 3/4 patients that had calvaria lesions (excluding the aforementioned skull-base lesion). All cases involving the calvarium were described as homogenous, well-defined lesions with gadolinium enhancement on T1-weighted MRI. On T2 sequences, the tumors appeared isointense. Only Subasinghe et al. and Jiang et al. included CT imaging, which revealed osteolytic destruction of the skull with intracranial extension, but without penetration of the meninges or brain parenchyma.[
Clinical management
Proper investigation into a primary site of cancer is essential in the workup of these patients and all articles referred to this in their clinical management. Ferraz et al. were not specific in their workup for this.[
For treatment, 3/5 studies performed en bloc resection with craniectomy at the site of the lesion. 1/5 performed palliative excision of the scalp, but there was no mention of rationale of palliative excision versus gross total resection.[
OUTCOMES
Outcomes in these few patients remain inconsistent, yet poor. 3/5 of these patients died due to liver failure (6 months,[
CONCLUSION
Purely intraosseous skull masses are uncommon. Benign and malignant lesions, including metastases, should be considered in the differential diagnosis of a skull mass. Due to their rarity, HCC-skull metastases are not often reported in the literature, and there exists a paucity of articles discussing their clinical presentation, radiological imaging, pathology, and clinical management. Furthermore, HCC-skull metastases presenting as a solitary skull lesion in patients without known concomitant cancer are exceedingly rare and have only been reported 5 times; this present case report brings that total to 6. Here, we characterized a case and summarized the existing data on solitary HCC-skull lesions in patients without known primary cancer.
Declaration of patient consent
Patient’s consent not required as patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
Acknowledgments
The authors acknowledge Stephen Lau for the histopathological images and associated figure captions.
References
1. Chen R, Gao Z, Wu X, Campbell JL, Zhang P, Chen B. Hepatocellular carcinoma presenting as thoracic spinal canal metastasis with no clinical primary foci: A report of a rare case and review of the literature. Oncol Lett. 2015. 10: 2333-6
2. Ferraz VR, Vitorino-Araújo JL, Sementilli L, Neto JF, Veiga JC. Lesion in scalp and skull as the first manifestation of hepatocellular carcinoma. Case Rep Neurol Med. 2016. 2016: 2897048
3. Hsieh CT, Sun JM, Tsai WC, Tsai TH, Chiang YH, Liu MY. Skull metastasis from hepatocellular carcinoma. Acta Neurochir (Wien). 2007. 149: 185-90
4. Jiang Y, Guo X, Yin J. Solitary skull metastasis as the first symptom of hepatocellular carcinoma: Case report and literature review. Neuropsychiatr Dis Treat. 2014. 10: 681-6
5. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology. 2017. 66: 510-7
6. Sadik KW, Dayoub H, Bonatti H. Superior sagittal sinus tumor eroding through the skull: An unfamiliar presentation of hepatocellular carcinoma and literature review. Case Rep Surg. 2019. 2019: 5945726
7. Shim YS, Ahn JY, Cho JH, Lee KS. Solitary skull metastasis as initial manifestation of hepatocellular carcinoma. World J Surg Oncol. 2008. 6: 66
8. Subasinghe D, Keppetiyagama CT, Sudasinghe H, Wadanamby S, Perera N, Sivaganesh S. Solitary scalp metastasis-a rare presentation of hepatocellular carcinoma. Ann Surg Innov Res. 2015. 9: 4
9. Stark AM, Eichmann T, Mehdorn HM. Skull metastases: Clinical features, differential diagnosis, and review of the literature. Surg Neurol. 2003. 60: 219-25 discussion 225-6
10. Trivedi P, Gupta A, Pasricha S, Agrawal G, Shah M. Isolated skull base metastasis as the first manifestation of hepatocellular carcinoma--a rare case report with review of literature. J Gastrointest Cancer. 2009. 40: 10-4