- College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia
- King Abdullah International Medical Research Center, Jeddah, Saudi Arabia
- Department of Neurosurgery, King Fahad General Hospital, Ministry of Health, Jeddah, Saudi Arabia
- Department of Pathology and Laboratory Medicine, King Abdulaziz Medical City, National Guard Health Affairs, Jeddah, Saudi Arabia.
Correspondence Address:
Abdulaziz M. Alghamdi, College of Medicine, King Saud Bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia.
DOI:10.25259/SNI_817_2023
Copyright: © 2024 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, transform, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Abdulaziz M. Alghamdi1,2, Taghreed A. Alsinani3, Alaa Samkari1,2,4. Solitary plasmacytoma of the calvarium: A successful management of a long-standing large lesion with a long-term follow-up. 26-Jan-2024;15:27
How to cite this URL: Abdulaziz M. Alghamdi1,2, Taghreed A. Alsinani3, Alaa Samkari1,2,4. Solitary plasmacytoma of the calvarium: A successful management of a long-standing large lesion with a long-term follow-up. 26-Jan-2024;15:27. Available from: https://surgicalneurologyint.com/surgicalint-articles/12721/
Abstract
Background: Solitary plasmacytoma of the calvarium (SPC), without evidence of multiple myeloma (MM), is extremely rare. We report a case of a long-standing large SPC that was treated successfully by surgical excision and adjuvant radiotherapy with a long follow-up period.
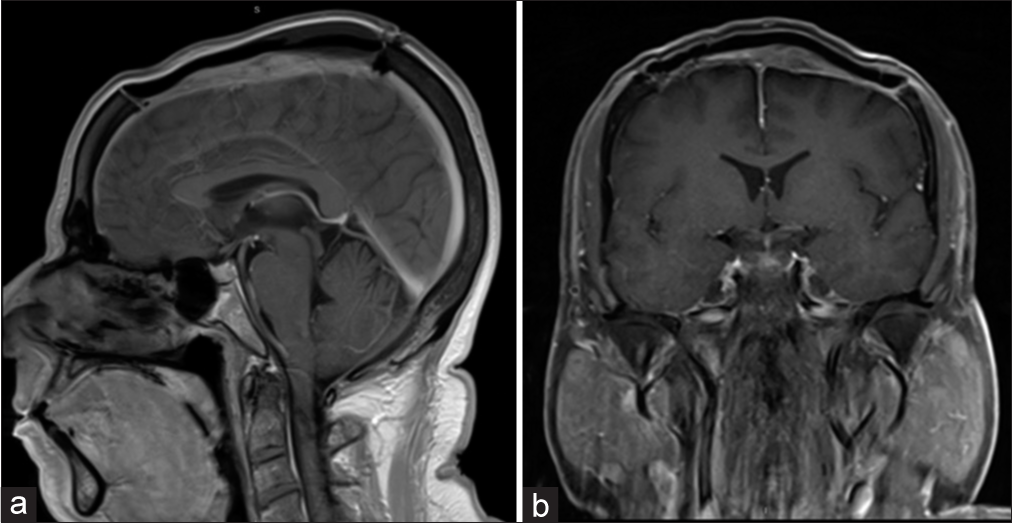
Case Description: A 58-year-old male patient presented with a 5-year history of painless skull swelling. On the physical examination, the mass was 6 × 6 cm in size, oval, not tender, and firm in consistency with normal skin color. A brain computed tomography scan showed a destructive skull lesion. A brain magnetic reasoning imaging (MRI) showed a large expansile lytic mass lesion arising from the skull vault in the frontal-parietal region with multiple internal septa. The patient underwent a craniectomy and excision of the lesion, followed by cranioplasty using methyl methacrylate. The final diagnosis was consistent with plasmacytoma based on the histopathological features. One month follow-up brain MRI showed no evidence of residual tumor. The skeletal survey and bone marrow biopsy did not reveal any evidence of MM. The patient was referred to medical oncology for further treatment and received radiation therapy. During nine years of clinical and radiological follow-up, there was no evidence of any metastasis or recurrence.
Conclusion: SPC is a rare disease with high rates of misdiagnosis. Careful evaluations are crucial to exclude systemic involvement. Surgical resection followed by radiotherapy may be adequate for the disease control. Close follow-up with regular lifelong examinations is important to avoid a generalized incurable disease.
Keywords: Calvarium, Case report, Multiple myeloma, Solitary plasmacytoma
INTRODUCTION
Osteolytic skull lesions are commonly observed in routine clinical work. One of these is plasma cell neoplasm, which arises from the proliferation of a single clone of B-cell lymphocytes. It produces a wide spectrum of disorders, ranging from solitary plasmacytoma (SP) to extremely malignant multiple myeloma (MM).[
CASE DESCRIPTION
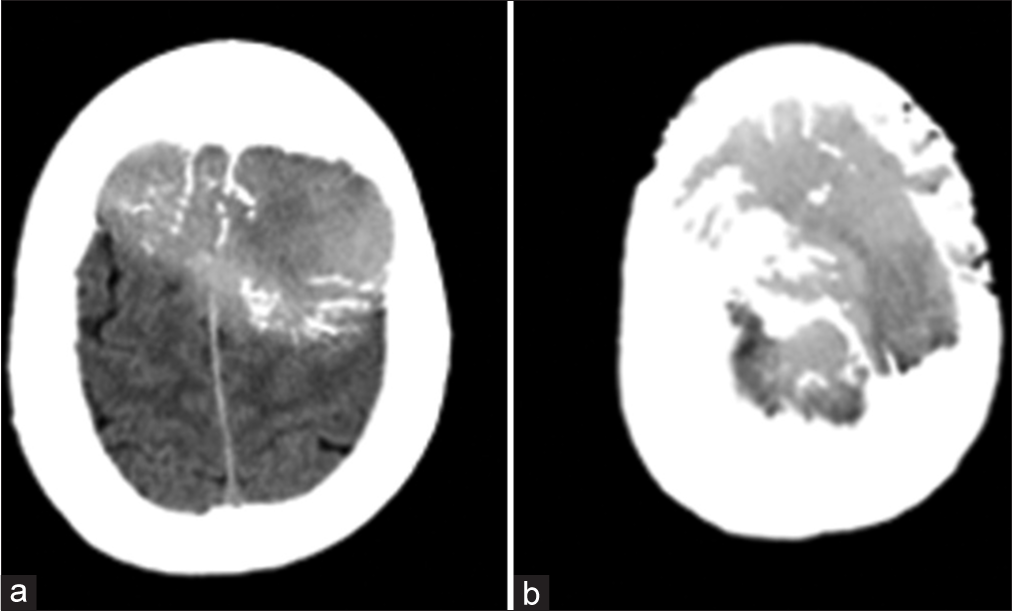
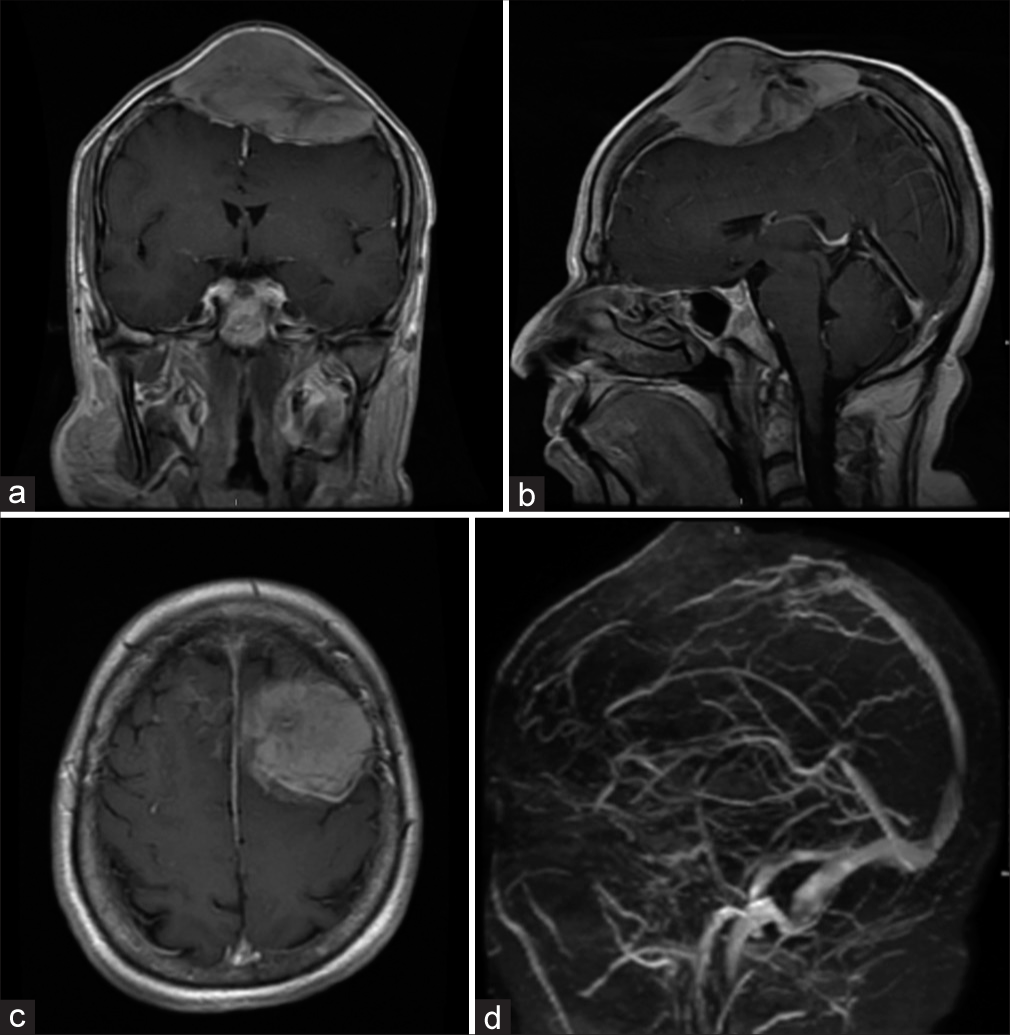
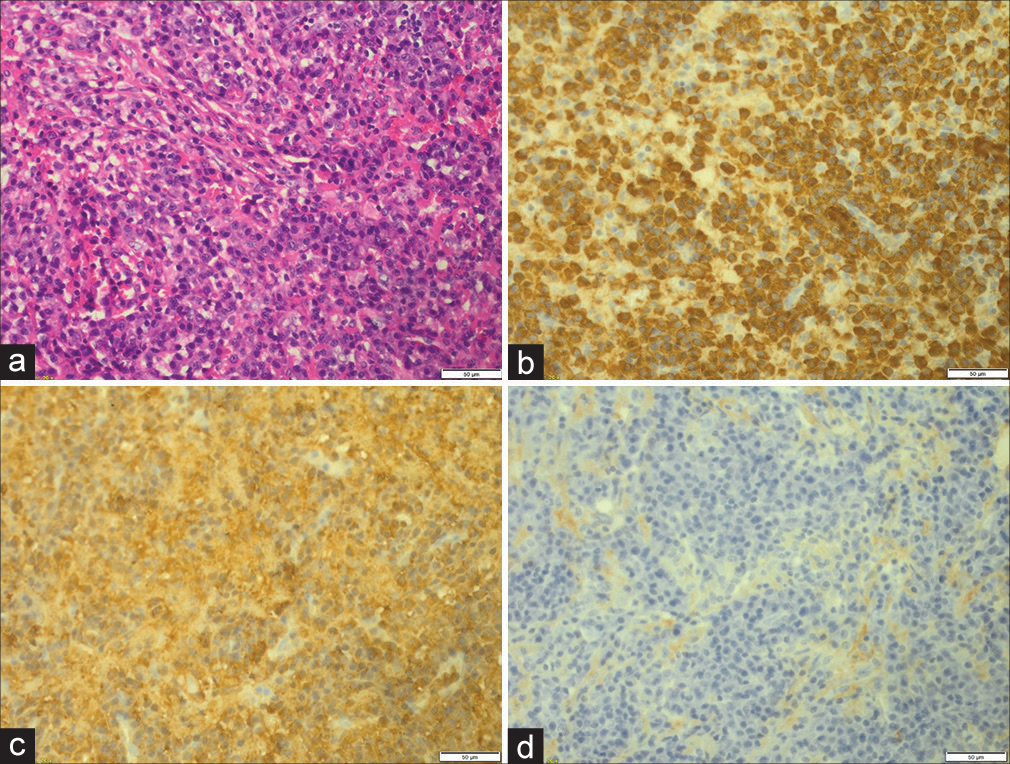
A 58-year-old male patient, who is not known to have any medical or surgical histories, presented in November 2014 to our hospital complaining of a painless skull swelling. He first noticed the skull swelling five years ago, and since then, he reported that it did not increase in size until he sustained a head trauma several months before the presentation. He claimed that the reason he had not sought medical attention for the past few years was because he was not bothered by the lesion. However, after the head trauma he sustained, the lesion got bigger, and he became worried and came to the hospital. He did not report having any neurological deficits. There was no history of weight loss, fever, or night sweating. He did not have a family history of malignancy. On the physical examination, the mass was 6 × 6 cm in size, oval, not tender, and firm in consistency with normal skin color. Other neurological examinations revealed intact functions without any subtle motor deficit. A brain computed tomography (CT) scan showed a destructive skull lesion that is causing spike-like projections that are invading the surrounding cortical bone [
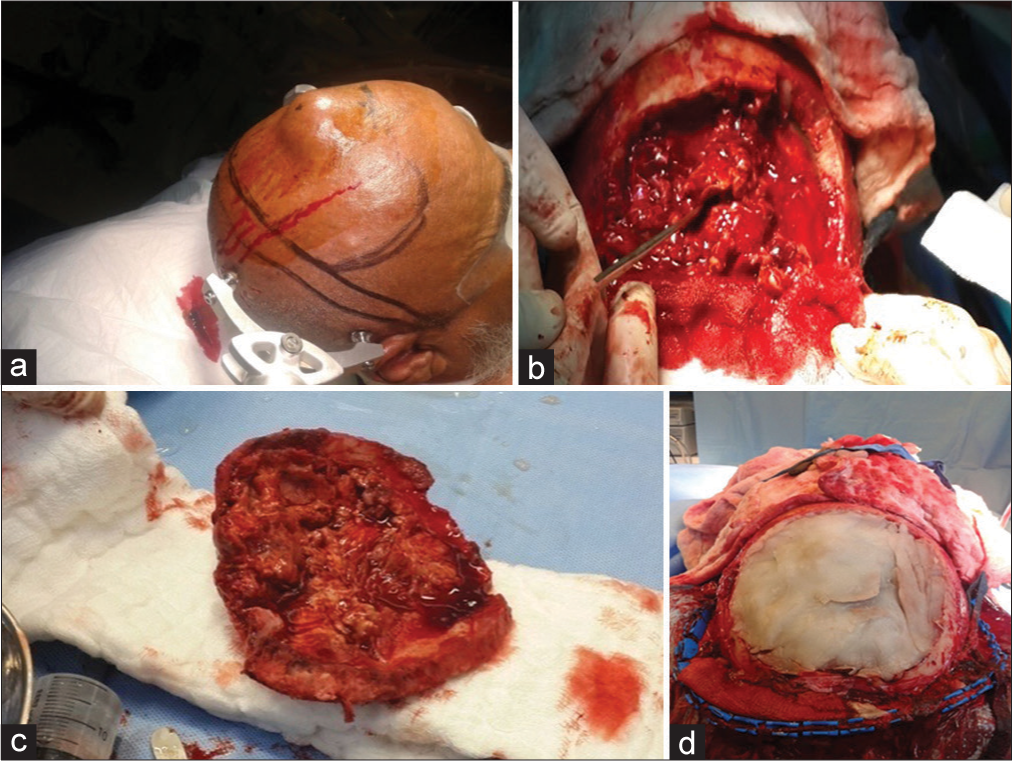
Following the lesion excision, a cranioplasty using methyl methacrylate was utilized [
DISCUSSION
SP is a rare malignancy and is characterized by malignant proliferation of monoclonal plasma cells.[
Limitations
This study has some limitations as it only included one case report of SPC. However, considering how rare this condition is, we aim to contribute to the literature to help understand these lesions better. Further studies with more cases of SPC to help understand the clinical features and predict the prognosis are recommended.
CONCLUSION
SPC is a rare disease. Clinical and imaging evaluations are of paramount importance to exclude systemic involvement. Careful surgical resection, if total, may be adequate for disease control. Nevertheless, close follow-up with regular lifelong examinations is important to avoid a generalized incurable disease.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
References
1. Barzenje DA, Kolstad A, Ghanima W, Holte H. Long-term outcome of patients with solitary plasmacytoma treated with radiotherapy: A population-based, single-center study with median follow-up of 13.7 years. Hematol Oncol. 2018. 36: 217-23
2. Du Preez JH, Branca EP. Plasmacytoma of the skull. Neurosurgery. 1991. 29: 902-6
3. Ellington TD, Henley SJ, Wilson RJ, Wu M, Richardson LC. Trends in solitary plasmacytoma, extramedullary plasmacytoma, and plasma cell myeloma incidence and myeloma mortality by racial-ethnic group, United States 2003-2016. Cancer Med. 2021. 10: 386-95
4. Fletcher CD, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC; 2002. p.
5. Provenzale JM, Schaefer P, Traweek ST, Ferry J, Moore JO, Friedman AH. Craniocerebral plasmacytoma: MR features. Am J Neuroradiol. 1997. 18: 389-92
6. Rajkumar SV, Kyle RA. Multiple myeloma: Diagnosis and treatment. Mayo Clin Proc. 2005. 80: 1371-82
7. Tanaka M, Shibui S, Nomura K, Nakanishi Y. Solitary plasmacytoma of the skull: A case report. Jpn J Clin Oncol. 1998. 28: 626-30
8. Webb M, Barrett C, Barrett S, van Rensburg JJ, Louw V. Cranial plasmacytoma: A case series and review of the literature. Indian J Hematol Blood Transfus. 2013. 29: 43-7
9. Wein RO, Popat SR, Doerr TD, Dutcher PO. Plasma cell tumors of the skull base: Four case reports and literature review. Skull Base. 2002. 12: 77-86