- Department of Neurosurgery, Salford Royal NHS Foundation Trust, Greater Manchester, United Kingdom.
- Department of Neurology, Salford Royal NHS Foundation Trust, Greater Manchester, United Kingdom.
- Department of Neurology, Manchester Centre for Genomic Medicine, St Mary’s Hospital, Greater Manchester, United Kingdom.
- Department of Cellular Pathology, Salford Royal NHS Foundation Trust, Greater Manchester, United Kingdom.
- Department of Radiology, Manchester University NHS Foundation Trust, Greater Manchester, United Kingdom.
Correspondence Address:
Md Tanvir Hasan
Department of Neurosurgery, Salford Royal NHS Foundation Trust, Greater Manchester, United Kingdom.
DOI:10.25259/SNI_35_2021
Copyright: © 2021 Surgical Neurology International This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.How to cite this article: Md Tanvir Hasan1, Subodh Patil1, Vanisha Chauhan2, David Gosal2, John Ealing3, Daniel Du Plessis4, Calvin Soh5, K. Joshi George1. Spinal cord compression from hypertrophic nerve roots in chronic inflammatory demyelinating polyradiculoneuropathy – A case report. 24-Mar-2021;12:114
How to cite this URL: Md Tanvir Hasan1, Subodh Patil1, Vanisha Chauhan2, David Gosal2, John Ealing3, Daniel Du Plessis4, Calvin Soh5, K. Joshi George1. Spinal cord compression from hypertrophic nerve roots in chronic inflammatory demyelinating polyradiculoneuropathy – A case report. 24-Mar-2021;12:114. Available from: https://surgicalneurologyint.com/surgicalint-articles/10665/
Abstract
Background: Spinal cord compression secondary to nerve root hypertrophy is often attributed to hereditary neuropathies. However, to avoid misdiagnosis, rare immune-mediated neuropathy such as chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) should not be overlooked. This report presents a case of multilevel nerve root hypertrophy leading to significant cord compression from CIDP.
Case Description: We report a 56-year-old gentleman with type two diabetes mellitus who presented with subacute cervical cord syndrome following a fall. Mixed upper and lower motor neuron features were noted on examination. Magnetic resonance imaging showed significant pan-spinal proximal nerve root hypertrophy, compressing the cervical spinal cord. Initial radiological opinion raised the possibility of neurofibromatosis type 1 (NF-1), but neurophysiology revealed both axonal and demyelinating changes that were etiologically non-specific. C6 root and sural nerve biopsies taken at cervical decompression displayed striking features suggestive for CIDP. Although NF-1 is the most observed condition associated with root hypertrophy, other important and potentially treatable differentials need to be entertained.
Conclusion: While rare, CIDP can cause significant spinal cord compression. Furthermore, clinical manifestations of CIDP can mimic those of inherited peripheral neuropathies. Neurologists and neurosurgeons should be aware of this condition to optimize subsequent therapeutic decision-making.
Keywords: Charcot-marie-tooth disease, Chronic inflammatory demyelinating polyradiculoneuropathy, Hypertrophic neuropathy, Neurofibromatosis
INTRODUCTION
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is immune-mediated neuropathy of peripheral nerves and nerve roots involving myelin sheath. The symptoms usually include symmetrical weakness of both proximal and distal muscles, sensory impairment, absent or diminished tendon reflexes, with signs of demyelination in nerve conduction studies, as well as in nerve biopsy specimens. These can be insidious, progressively increasing over 2 months with relapsing or chronic and progressive phase.[
CASE DESCRIPTION
A 56-year-old gentleman with a background history of type 2 diabetes mellitus (6 years of sound control and no associated complications), hypertension, and medically controlled hyperthyroidism presented to his local hospital with a 6-week history of progressive lower limb weakness, voiding difficulties, constipation, and burning paraesthesia of the soles of his feet, after a minor fall and head injury at work. He denied radicular pains in upper or lower limbs, or preceding neurological difficulties. He was otherwise systemically well. Due to the sphincter disturbance, he underwent emergency imaging of the lumbar spine which showed massive thickening of the cauda equina and lumbar roots and prompted imaging of the rest of his spine. This showed a similar picture with superimposed degenerative changes involving the cervical spine with evidence of midcervical cord compression due to a combination of discogenic disease and nerve root hypertrophy [
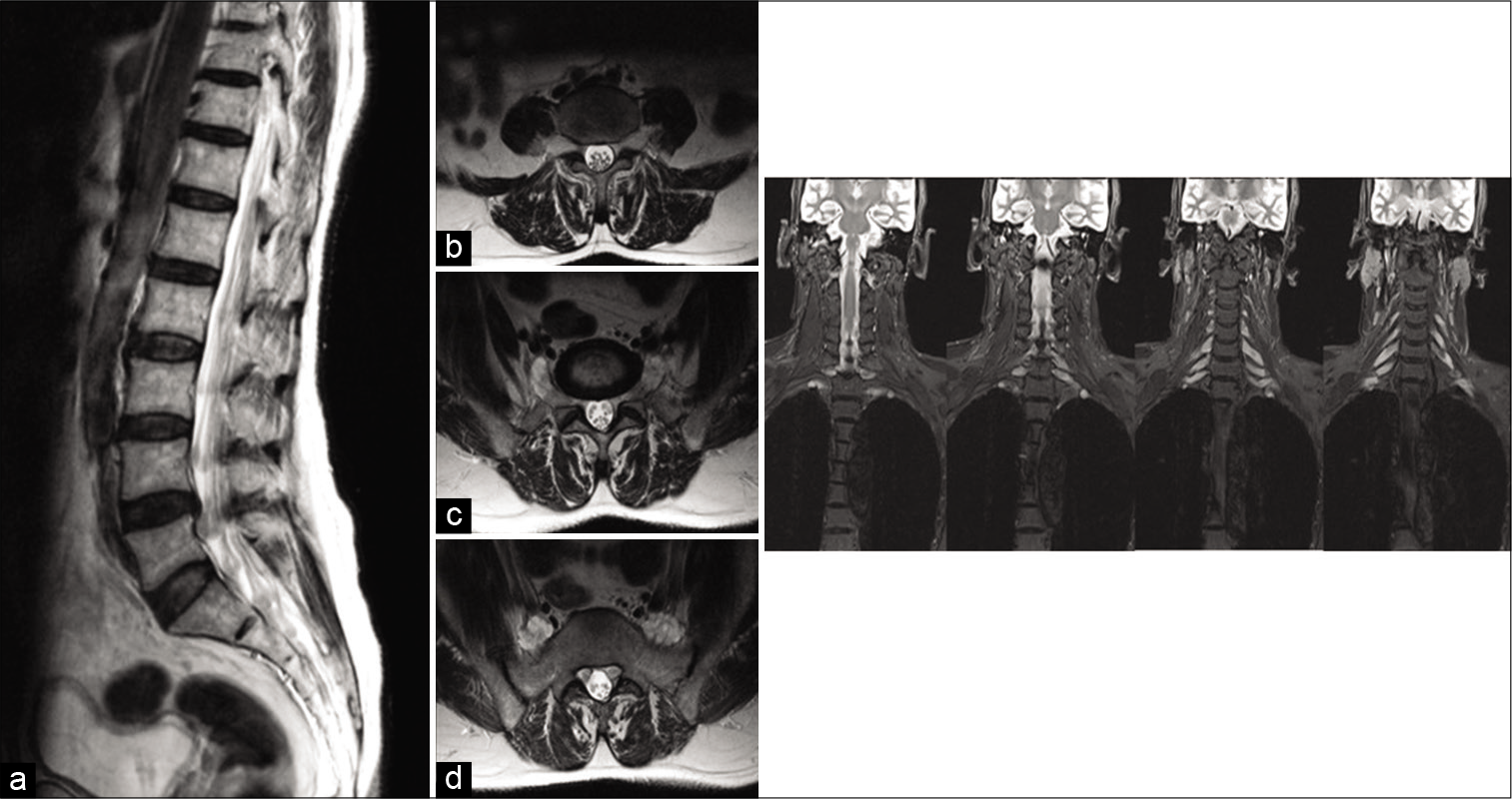
Figure 1:
Left: Lumbar nerve root involvement. (a) Sagittal T2 (off midline) shows multiple intradural nerve root nodular lesions approaching the exit foramina. (b) Axial T2 through L3 foramina shows enlarged L3 roots at the exit foramina with some thickening of the intradural cauda equina roots. (c) Axial T2 through L4 exit foramina shows thickened L4 roots extending outwards from foramina, while intradural cauda equina roots are thickened. (d) Axial T2 through S1 segment of sacrum shows lobulated L5 roots ventral to sacral alar, while S1 nerve roots at lateral recesses are enlarged. Right: Coronal STIR of the brachial plexus in sequential images from posterior to anterior. All the cervical roots are thickened and hyperintense. The first image shows the intradural components in the spinal canal.
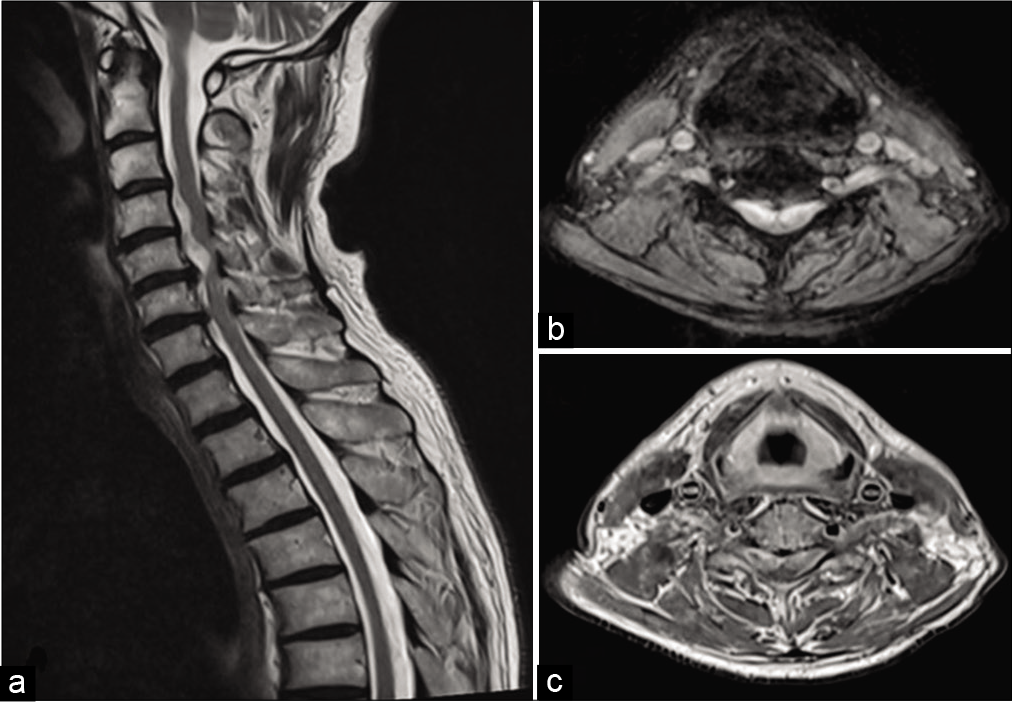
Figure 2:
(a) Sagittal T2 shows hyperintense segmental lesions ventrolateral to the cervical cord at C4 and C5 vertebral levels compressing the cervical cord. (b) Axial T2 and (c) axial post-Gadolinium T1 at lower border of C5 vertebral level show bilateral C6 root lesions compressing the cervical cord which lies in the dorsal midline.
Given the massive root hypertrophy, an initial diagnosis of neurofibromatosis type 1 (NF-1) was entertained. The patient was referred urgently to the regional NF-1 unit in Greater Manchester. On examination, no cutaneous stigmata of NF-1 could be appreciated, nor was there evidence of peripheral nerve hypertrophy. Upper and lower motor neuron signs were present with evidence of mild symmetrical wasting of distal lower limb muscles, predominant pyramidal distribution of weakness in the lower limbs and to a milder extent in the upper limbs with global areflexia and extensor plantar responses. Vibration sense was impaired to the anterior superior iliac spine with a reduction in pinprick to the mid-thighs and altered sensation to T8 with some additional non-specific sensory disturbance over the ulnar distributions of the hands bilaterally. He was unable to stand or walk unaided. It was felt that the presentation was atypical for NF-1, and an opinion was sought from the regional neuromuscular service while arrangements were made for cervical decompression. Genetic testing for NF-1 was taken.
The neuromuscular service’s subsequent review revealed similar examination findings [
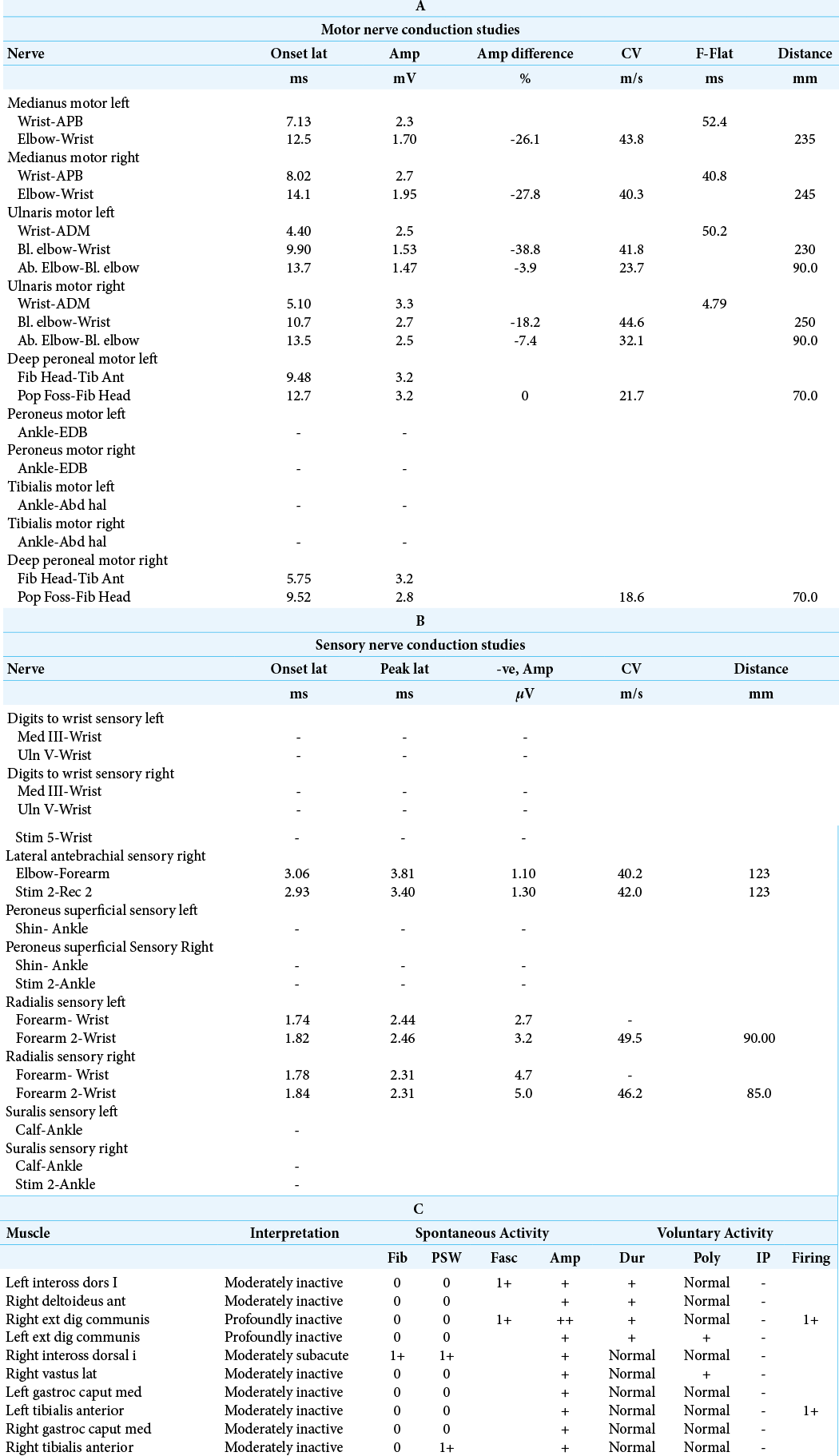
Table 1:
(A) Median motor conductions on both sides show severe distal motor latency delay and reduced motor potential amplitude, motor potentials are temporarily dispersed. Forearm motor conduction slowing on both sides and F latencies show moderate delay in latency. Ulnar motor conduction on both sides show delay in distal motor latency and reduced motor potential amplitude, dispersed particularly proximally, mild forearm motor conduction slowing, and moderate motor conduction slowing across the elbow on both sides. Moderate to severe reduction in lower limb motor conduction (common peronial? Tibialis) (B) Median digit III and ulnar digit V-no recordable sensory potentials. Radial sensory conduction on both sides-reduced sensory potential amplitude and no significant sensory conduction slowing. Lateral antebrachial cutaneous nerve sensory conduction on the right side-markedly reduced sensory potential with sensory conduction slowing. In both lower limbs show no recordable sensory potential. (C) Needle EMG done in tibialis anterior and gastrocnemius on both sides as well as right vastus lateralis in the lower limbs; includes first dorsal interosseous. EDC on both sides in the upper limbs and right deltoid. Active denervation is noted in right first dorsal interosseous and to a lesser degree in the right tibialis anterior. Prominent chronic denervation is noted in all these muscles and the changes are relatively more prominent in bilateral extensor digitorum communis in the upper limb and the left tibialis anterior in the lower limb.
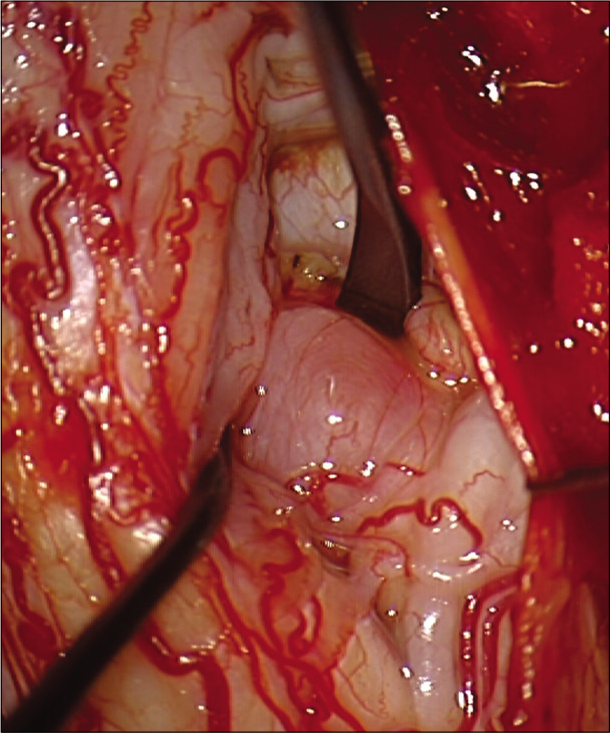
Surgery was performed in standard prone position under total intravenous anesthesia with neurophysiology monitoring. A C4-C6 laminoplasty was performed. After opening the dura mater, the motor evoked potentials amplitudes in the lower limbs improved. The arachnoid was thickened, and large tumor-like swellings involving the C5 and C6 nerves bilaterally, ventral to cord could be appreciated. These appeared to arise from the motor roots as stimulated by the intraoperative nerve stimulator except the left C6 nerve root which was subsequently biopsied. A frozen section demonstrated dispersed large, myelinated axons throughout the lesional tissue, contradicting a diagnosis of a peripheral nerve sheath tumor. Instead of debulking, an expansive duraplasty, followed by expansive laminoplasty was therefore performed. A left sural nerve biopsy was also taken.
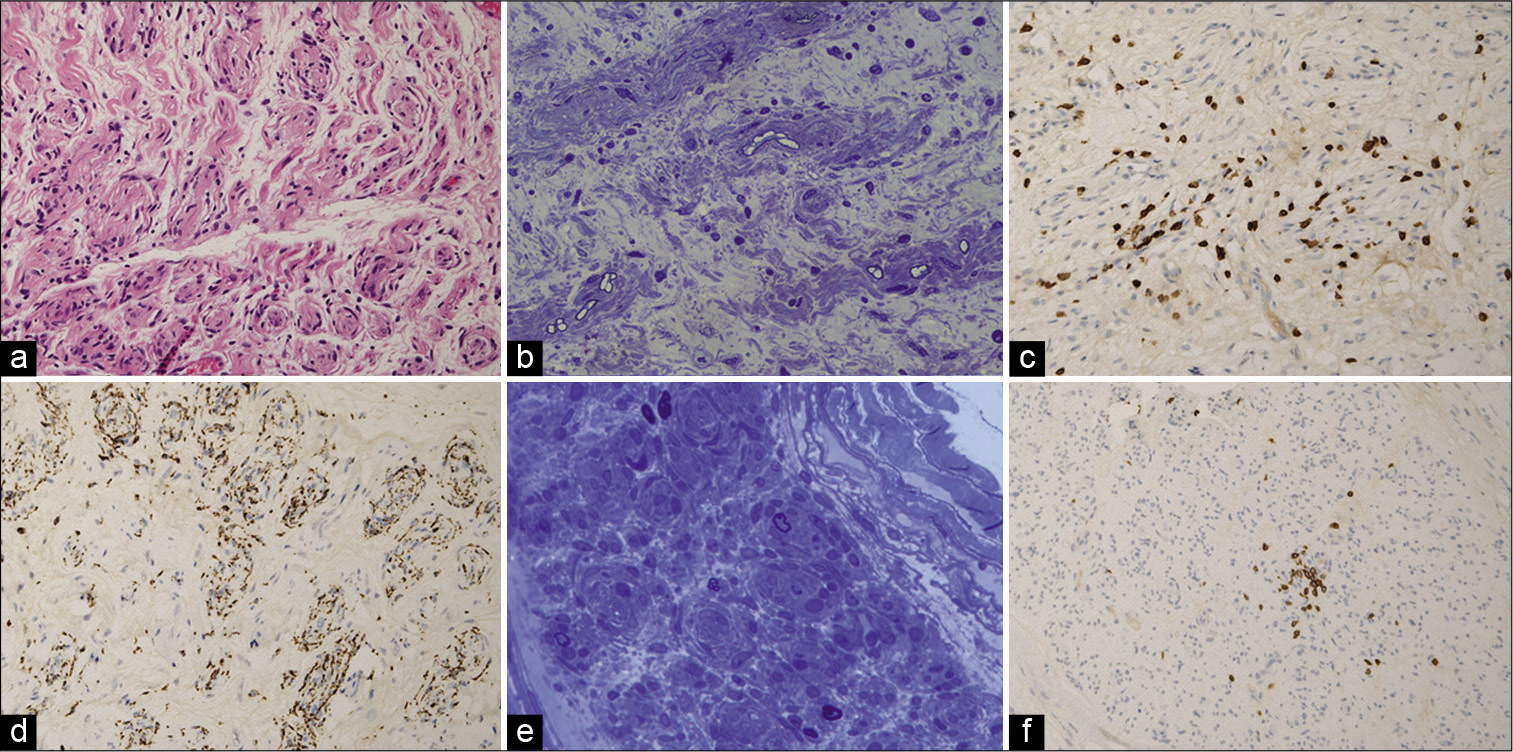
Histopathology of both the root and nerve biopsies showed striking features classical for a chronic inflammatory demyelinating neuropathy with marked loss of large, myelinated fibers, endoneurial edema, thinly remyelinated axons, Schwann cell onion skinning, and T cell inflammatory infiltrates [
Figure 4:
C6 nerve root biopsy: (a) H&E (hematoxylin and eosin) stained section showing endoneurial edema and a minifascicular architecture (in longitudinal and transverse profile) due to Schwann cell proliferation militating against a neoplastic or infectious process. (b) Semi-thin resin section (toluidine blue stained) showing a marked reduction in the density of large myelinated fibers and a prominent edematous stroma. The pathology is further characterized by thinly myelinated fibers surrounded by multi-layered sheaths of Schwann cells, which all favor a demyelinating process. (c) CD3 immunostaining for T lymphocytes showed prominent focal endoneurial lymphocytic activity. (d) CD68 immunostaining highlighted phagocytic activity co-localized to the cords of Schwann cells. Sural nerve biopsy: (e) Semi-thin resin section (toluidine blue stained) showing striking loss of large myelinated fibers, endoneurial edema and concentric Schwann cell proliferation. (f) CD3 immunostaining again showing a focal endoneurial inflammatory infiltrate of T-lymphocytes.
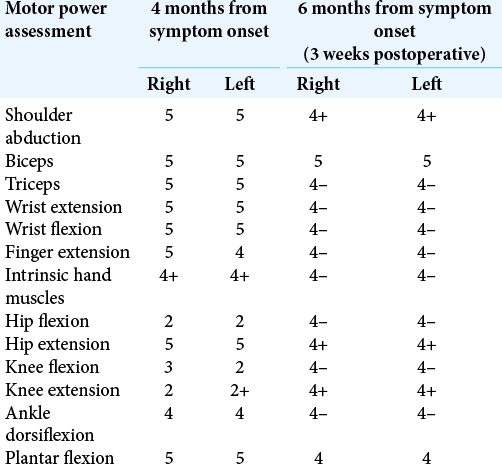
Six weeks after surgical decompression, the patient commenced monthly pulses of immunoglobulin therapy and is making slow and steady improvements to the point where he is mobilizing independently [
Table 2:
MRC scores interpretation: (0) no contraction; (1) palpable contraction but no visible movement; (2) movement without gravity; (3) movement against gravity; (4) movement against a resistance lower than the resistance overcome by the healthy side; (5) movement against a resistance equal to the maximum resistance overcome by the healthy side.
DISCUSSION
This 56-year-old gentleman presented with subacute cervical myelopathy caused by a combination of degenerative spinal disease and proximal nerve root enlargement. Proximal nerve root enlargement has a broad differential diagnosis, of which CIDP is an important etiology.[
Magnetic resonance imaging (MRI) remains an essential radiological diagnostic modality. CIDP may show hypertrophy of the cervical and lumbar spinal roots, brachial and lumbosacral plexuses, and contrast enhancement in active disease.[
In contrast, cervical cord compression by multilevel segmental neurofibromas is frequently seen[
To differentiate the cause of hypertrophy, nerve conduction study and electromyography were performed. These also evaluate the presence, degree and pattern of conduction slowing along motor and sensory nerves in proximal and distal segments which indirectly provides evidence of demyelination, classically seen in CIDP and CMT1. Reduction in compound muscle action potential for motor nerves and sensory nerve action potential for sensory nerves represents the degree of axonal damage and loss of fibers. In our patient, there was electrophysiological evidence of underlying mixed demyelinating and axonal generalized large fiber polyradiculoneuropathy [
With ongoing diagnostic dilemma, the neurosurgical team proceeded with decompressive surgery for severe cord compression, keeping in mind there may be concomitant hereditary nerve root hypertrophy. Subsequent biopsy (C6 nerve root and sural nerve) revealed thinly myelinated large axons surrounded by multi-layered sheaths of Schwann cells traversing an edematous expanded stromal background, with endoneurial T lymphocyte-mediated inflammatory activity, and phagocytic activity mediated either by macrophages and/or Schwann cells [
In this case, we theorize that the acquired narrowed cervical spinal canal through disc disease and nerve root hypertrophy left little room for compensation following this gentleman’s fall, resulting in subacute cervical myelopathy which ultimately led to his diagnosis of CIDP. This case further highlight that the degree of nerve enlargement on MRI imaging does not correlate with clinical symptoms, as before his fall, the gentleman was asymptomatic.
CONCLUSION
CIDP can possess diagnostic uncertainty with atypical presentations and shares similarities with genetic disorders such as neurofibromatosis and Charcot-Marie-Tooth disease. This case illustrates a relatively rare presentation of CIDP in the setting of spinal cord compression and the importance of keeping a broad differential diagnosis, taking into careful consideration the clinical, neurological, and pathological findings.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
1. Austin JH. Recurrent polyneuropathies and their corticosteroid treatment: With five-year observations of a placebo-controlled case treated with corticotrophin, cortisone, and prednisone. Brain. 1958. 81: 157-92
2. Bril V, Blanchette CM, Noone JM, Runken MC, Gelinas D, Russell JW. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J Diabetes Complications. 2016. 30: 1401-7
3. Curtis-Lopez CM, Soh C, Ealing J, Evans DG, Wright EM, Vassallo G. Clinical and neuroradiological characterisation of spinal lesions in adults with Neurofibromatosis Type 1. J Clin Neurosci. 2020. 77: 98-105
4. Drouet A, Wolkenstein P, Lefaucheur JP, Pinson S, Combemale P, Gherardi RK. Neurofibromatosis 1-associated neuropathies: A reappraisal. Brain. 2004. 127: 1993-2009
5. Duggins AJ, Mcleod JG, Pollard JD, Davies L, Yang F, Thompson EO. Spinal root and plexus hypertrophy in chronic inflammatory demyelinating polyneuropathy. Brain. 1999. 122: 1383-90
6. Eggermann K, Gess B, Häusler M, Weis J, Hahn A, Kurth I. Hereditary neuropathies. Dtsch Arztebl Int. 2018. 115: 91-7
7. Evans MR, Laurá M, Chandrashekar H, Reilly MM. Cervical spinal cord compression complicating the clinical course of Charcot-Marie-Tooth Type 1. BMJ Case Rep. 2015. 2015: bcr2015213486
8. Fatehi F, Nafissi S, Basiri K, Amiri M, Soltanzadeh A. Chronic inflammatory demyelinating polyneuropathy associated with diabetes mellitus. J Res Med Sci. 2013. 18: 438-41
9. Hughes RA, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2006. 11: 30-46
10. Khadilkar SV, Yadav RS, Soni G. A practical approach to enlargement of nerves, plexuses and roots. Pract Neurol. 2015. 15: 105-15
11. Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005. 352: 1343-56
12. Mathey EK, Park SB, Hughes RA, Pollard JD, Armati PJ, Barnett MH. Chronic inflammatory demyelinating polyradiculoneuropathy: From pathology to phenotype. J Neurol Neurosurg Psychiatry. 2015. 86: 973-85
13. Neto FJ, Filho EN, Miranda FC, Rosemberg LA, Santos DC, Taneja AK, Demystifying MR. Neurography of the lumbosacral plexus: From protocols to pathologies. Biomed Res Int. 2018. 2018: 9608947
14. Oudeman J, Eftimov F, Strijkers GJ, Schneiders JJ, Roosendaal SD, Engbersen MP. Diagnostic accuracy of MRI and ultrasound in chronic immune-mediated neuropathies. Neurology. 2020. 94: e62-74
15. Pareyson D. Differential diagnosis of charcot-marie-tooth disease and related neuropathies. Neurol Sci. 2004. 25: 72-82
16. Rajabally YA, Peric S, Cobeljic M, Afzal S, Bozovic I, Palibrk A. Chronic inflammatory demyelinating polyneuropathy associated with diabetes: A European multicentre comparative reappraisal. J Neurol Neurosurg Psychiatry. 2020. 91: 1100-4
17. . Report from an Ad Hoc subcommittee of the American academy of neurology AIDS task force. Neurology. 1991. 41: 617-8
18. Rezania PP, Frank BS, Wollmann R. Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) with hypertrophic spinal radiculopathy mimicking neurofibromatosis. Acta Neuropathol. 2003. 105: 185-8
19. Said G. Chronic inflammatory demyelinating polyneuropathy. Neuromuscul Disord. 2006. 16: 293-303
20. Shibuya K, Sugiyama A, Ito S, Misawa S, Sekiguchi Y, Mitsuma S. Reconstruction magnetic resonance neurography in chronic inflammatory demyelinating polyneuropathy. Ann Neurol. 2015. 77: 333-7
21. Staff NP, Figueroa JJ, Parisi JE, Klein CJ. Hypertrophic nerves producing myelopathy in fulminant cidp. Neurology. 2010. 75: 750
22. Tanaka K, Mori N, Yokota Y, Suenaga T. MRI of the cervical nerve roots in the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy: A single-institution, retrospective case-control study. BMJ Open. 2013. 3: e003443
23. Uncini A, de Angelis MV, di Muzio A, Callegarini C, Ciucci G, Antonini G. Chronic inflammatory demyelinating polyneuropathy in diabetics: Motor conductions are important in the differential diagnosis with diabetic polyneuropathy. Clin Neurophysiol. 1999. 110: 705-11
24. Vallat JM, Sommer C, Magy L. Chronic inflammatory demyelinating polyradiculoneuropathy: Diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol. 2010. 9: 402-12
25. Waqar M, Huson S, Evans DG, Ealing J, Karabatsou K, George KJ. C2 neurofibromas in neurofibromatosis Type 1: Genetic and imaging characteristics. J Neurosurg Spine. 2019. 30: 126-32